Classes of Chemical Preservation
Chemical preservation extends the shelf-life of the food by inhibiting endogenous enzymes, microorganisms, and deteriorative reactions such as lipid oxidation. Chemicals added are regulated by the Canadian Food Inspection Agency (CFIA) as additives and fall under category 11 – preservatives, which are subdivided into 4 classes: 1) curing agents, 2) antibacterial, 3) antifungal, and 4) antioxidant. The first three classes prevent microbial growth, while the fourth inhibits oxidation and prevents the consumption of free radicals. Chemical preservatives may indirectly inhibit spoilage by modifying intrinsic parameters (pH, redox potential (aerobic or anaerobic), or water activity (aw)) or by directly inhibiting spoilage enzymes or microbial growth.

Class 1-3 Preservatives – Microbial Growth Inhibition
Curing covers any food preservation technique that adds ionic molecules (salts) to draw moisture from the food by osmosis and is used to preserve meat, fish and vegetables. Dry curing rubs salt on the surface or mixes it into foods (sausage), while wet curing submerges food in brine containing dissolved salts in water, or the brine is injected into the intact muscle. Corning uses table or sea salt (NaCl or KCl) to inhibit microbial growth by plasmolysis (water migration out of the microbe cytoplasm).

Bacteria tolerate different salt concentrations; for example, Samonella is inhibited with 3% NaCl, while 20% is required to inhibit the growth of Staphylococcus. Pickling differs from curing as it uses sugars and salts to create the osmotic gradient. A key curing element is the decrease in the food’s water activity (aw), an intrinsic parameter. However, some salts exert additional antimicrobial effects, including nitrites and nitrates.
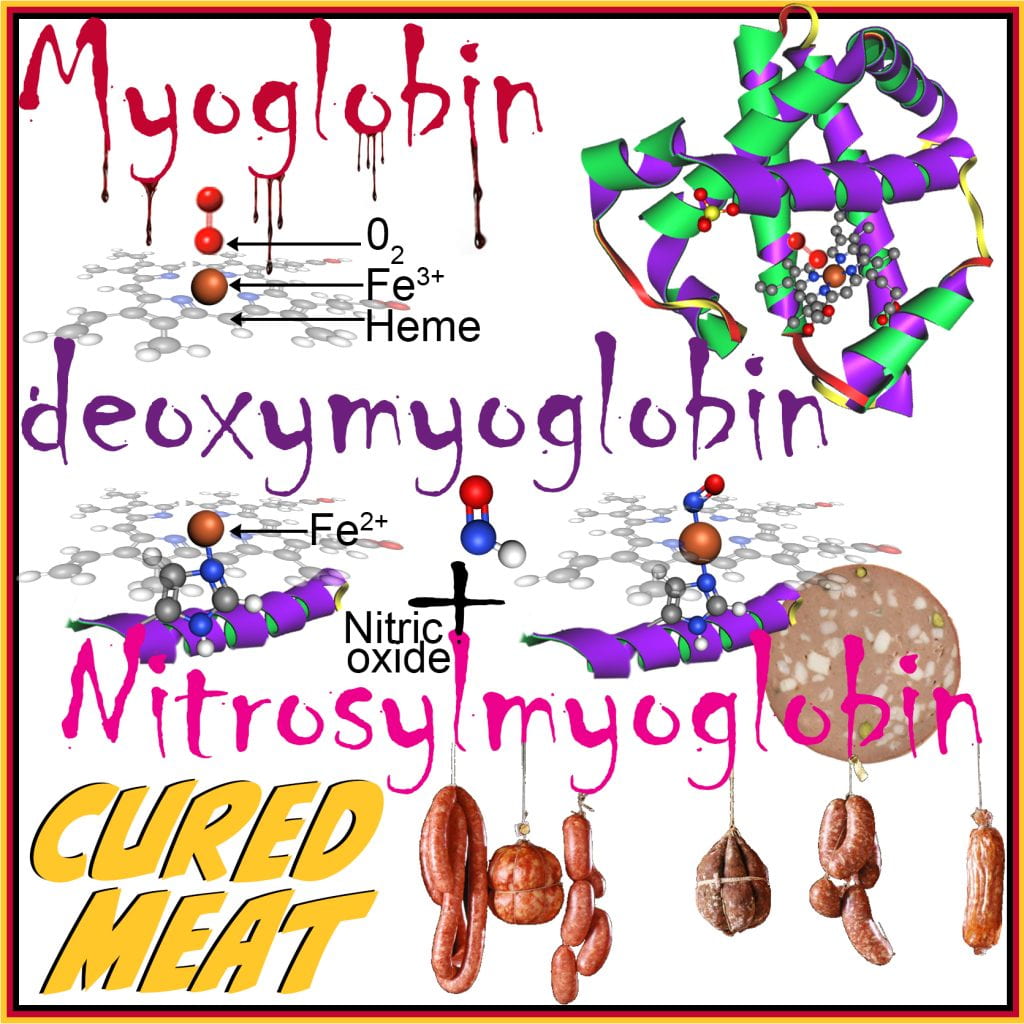
Curing salt contains >90% NaCl and the remainder is nitrites (and potentially nitrates, but they have been banned in some foods, for example, bacon, due to the formation of nitrosamine, a potent carcinogen that forms at high temperatures), and is a common additive in processed meats, which inhibits the growth of pathogens and spoilage bacteria by preventing the germination of C. Botulinum and the growth of foodborne pathogens, including Listeria monocytogenes, Escherichia coli, and Micrococcus spp, by affecting oxygen uptake, oxidative phosphorylation, and critical enzymes in bacterial metabolism. Nitrites inhibit growth at much lower concentrations than NaCl and form complexes with myoglobin to form nitrosylmyoglobin, giving the cured meat its pink color.
Sorbic acid and its salts, including sodium, potassium, and calcium sorbates and toxic to mold, yeast and fungi at concentrations as low as 0.025% and are commonly added to processed foods. Sulfites naturally occur in some foods and are added to processed foods to prevent microbial growth and enzymatic and non-enzymatic (oxidative) browning of cut fruits and vegetables, especially important for frozen French fries and other sliced vegetables. Sulfiting agents include sodium sulfite, potassium bisulfite, sulfurous acid and sulfur dioxide. Unfortinualy, sulfites also affect the growth of probiotic microorganisms in the gut and cause sensitivities in some individuals. Sulfites are used in dried fruits, such as coconut and raisins, in dried herbs, spices, teas and in many condiments (ketchup, mustard, relish). Sulfates are not used in foods but in the detergent sodium laurel sulfate commonly used in shampoo. Bacteriocins, such as nicin, produced by Lactococcus lactis, is a polycyclic antibacterial peptide that inhibits the growth of Gram-positive spoilage and pathogenic bacteria.
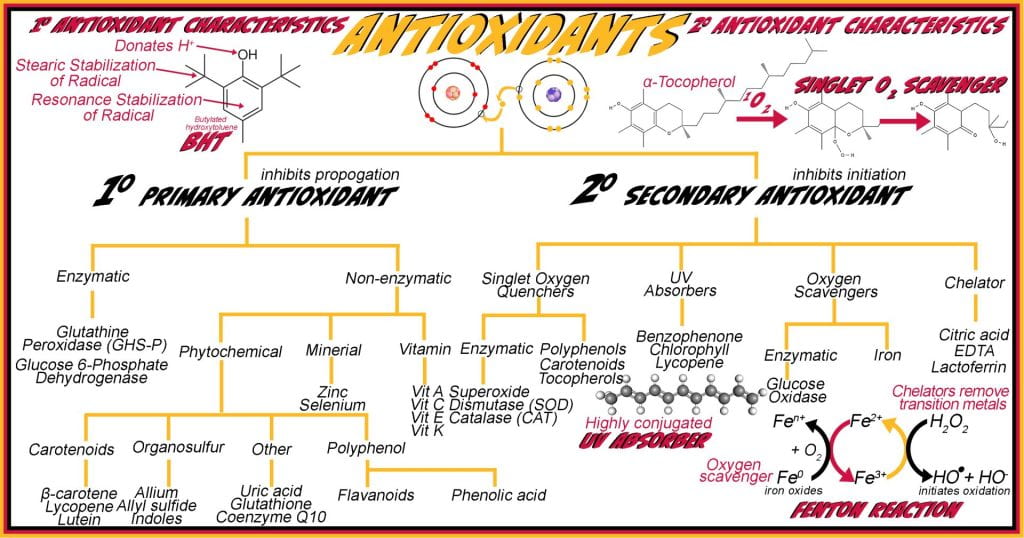
Class 4 Preservatives: Antioxidant
Radicals are undesirable in foods and toxic to living organisms; in both cases, antioxidants slow radical formation or eliminate radicals after induction. Primary 1o antioxidants donate hydrogen to a lipid radical, terminating its propagation, and the radical antioxidant then forms a stable compound breaking the cascade. Secondary 2o antioxidants inhibit the formation of the initial free radical, slowing radical initiation. Both 1o and 2o antioxidants are used endogenously as Class 4 preservatives in food systems. Tocopherols, butylated hydroxytoluene (BHT), and ascorbic acid are 1o antioxidants, while citric acid, 2o antioxidants, prevent oxidation by chelating iron involved in Fenton reaction.