Caseins Proteins

Milk proteins are subdivided into casein and whey proteins, where whey proteins exist as hydrated monomers-octamers while casein proteins conglomerate into casein micelles that are unique for each species; however, due to the importance of dairy products from bovine milk, its micelle will be the focus herein. Casein micelles are highly-hydrated, polydispersed discrete phosphoprotein/calcium phosphate particles between 80 – 400 nm, forming a stable suspension of nanoparticles. Specific to bovine milk, casein represents 80% of the protein and contains four fractions, alpahs1-, alphas2-, beta- and kappa caseins in a 4:1:3.5:1.5 ratio.1 Casein micelles do not adapt a supramolecular morphology of classical micelles, where the hydrophilic heads orientate towards the aqueous phase, and the hydrophobic tails orientate inward; instead alphas1-, alphas2-, beta-caseins aggregate between phosphorylation sites and salt bridges of calcium forming nanoclusters of calcium phosphate and are coated with k-casein that imparts surface stabilization in an aqueous phase.2
The AA sequence SerP-SerP-SerP-X-SerP in as2– and b-caseins are referred to as phosphate centers that bind calcium, well-exceeding calcium’s solubility limit in water.2 Proline, on alphas-1 casein, is restricted entirely to the hydrophobic ends of the protein and is separated by a polar region containing seven of eight phosphate groups that form salts with calcium. The proline’s cyclic nature restricts the angle and rotation of the peptide backbone, preventing the secondary structure from forming, leading to a primarily molten configuration and large blocks of hydrophobic AAs.
Calcium interacts strongly with the negatively-charged N-terminus of alphas2 casein, which localizes the positive charge at the opposite C-terminus. beta-casein is amphiphilic, such that the N-terminus is highly charged, able to interact with calcium, while the C-terminus is hydrophobic. With only a single phosphorylation site, k-casein does not interact with calcium; however, the C-terminus beyond AA residue Phe105-Met106 is highly glycosylated and hydrophilic.2
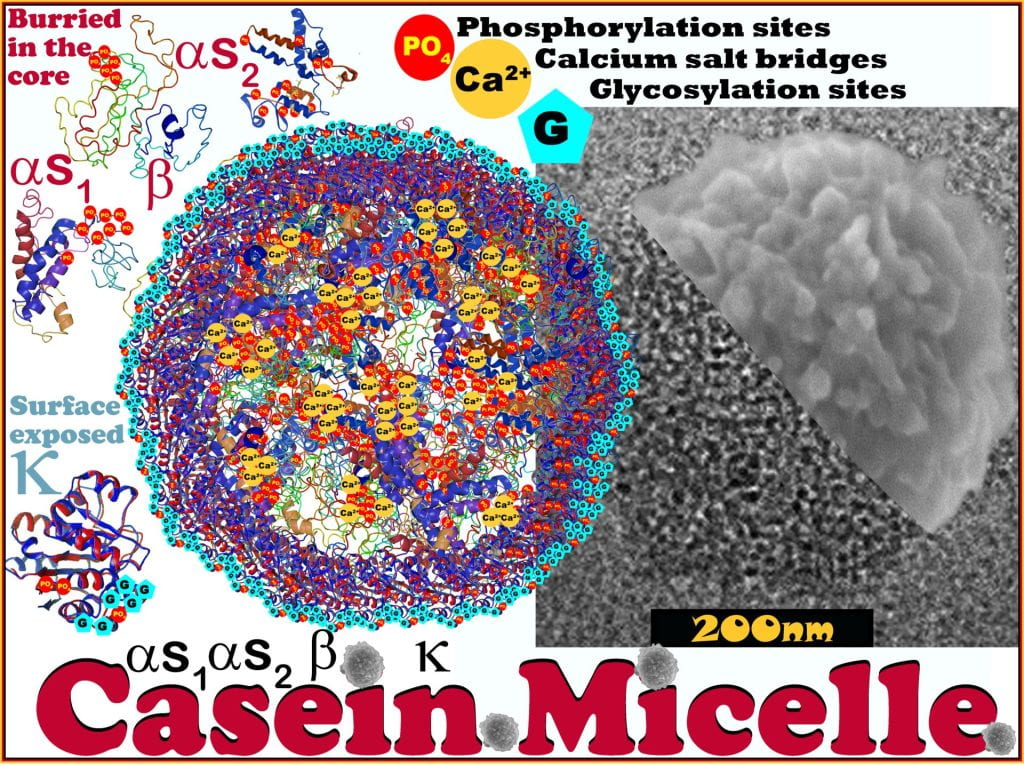
Like the other caseins, k-casein has large blocks of hydrophobic AAs; however, they are primarily disturbed before Phe105-Met106. This uneven AA distribution allows k-casein selectively coat the surface of the casein micelle concealing its hydrophobic tail from the water towards positioning it towards the protein clusters’ surface while orientating the glycosylated tail into the aqueous phase where it extends ~40 nm – termed the ‘hairy layer.’ The hairy layer imparts steric stabilization and weak electrostatic repulsion (at pH 7 z-potential ~ 20 mV) between micelles.2 The casein micelle is the most elegantly designed nanocarrier, increasing protein solubility and calcium bioaccessibility, making the superfood – milk.
Evolution of Bovine Caseins Proteins
The evolutionary adaptations of milk are remarkable, considering calf physiology and nutrition. At birth, the calf’s rumen is underdeveloped, resulting in muscular folds that become less prominent upon development. The temporary esophageal grove results from the reflective closure, which bypass milk directly into the abomasum over the first weeks of life, which acts as the stomach responsible for digesting nutrients.3 Moreover, it is there that the remarkable evolutionary advantage is obvious. For the first two weeks of life, the calf’s abomasum is more acidified in the fasted state (pH 1-2), and upon suckling, the milk buffers the gastric contents to a pH of ~6.4 The high pH and presence of milk induce secretion of abomasal acid, which slowly acidify its contents during proteolysis.4 For all mammalian neonates that produce chymosin, it is a newborn-specific gastric peptidase, and after birth, the highest amounts of the zymogen, prochymosin, are produced and then rapidly decline in concentration over the first two weeks of life. Upon exposure to an acidic environment, prochymosin forms chymosin, the active aspartic protease similar to other digestive proteases. However, unlike most digestive proteases, chymosin activity has a significantly higher optimum pH near ~5.8, compared to pepsin (optimum pH 2-5), with a lower overall activity but a greater affinity to cleave the Phe105-Met106 bond of k-casein – removing the hairy layer. Both pepsin and chymosin preferentially cleave before or after aromatic AAs phenylalanine, tyrosine, tryptophan and leucine, with chymosin being more substrate-specific than pepsin.5 Neonate pH is better suited to favor the activity of chymosin over pepsin and plays a significant role in neutralizing the charge and stearic repulsion induced by k-casein. The pH drop following milk consumption induces aggregation as the hairy layer is cleaved at the Phe105-Met106 bond of k-casein.6 As the pH decrease from physiological pH, protein solubility decreases, and at pH 4.6, caseins become insoluble and form a solid in the abomasum.2 While peptidase hydrolyzes numerous AA sites of proteins and produces small bioavailable peptides and AA; instead, the biological gain of chymosin is not to ‘digest’ the protein; instead, it coagulates the fresh milk into curdles of casein or cheese.
Selective pressure led to the continuous coevolution of casein proteins and chymosin; functional chymosin was not preserved in all mammalian clades, as is the case with humans, and the clade loss was associated with species that transfer immunoglobulins across the placenta.7 It is postulated that animals that cannot effectively transport immunoglobins across the placenta must prevent proteolytic degradation to the milk immunoglobulins.5,7 170 to 60 Ma saw the most rapid change in the k-casein AA sequence, and after 60 Ma, the structure was relatively stable.6 At 166 Ma, k-casein diverged between egg-laying mammals and therian mammals, including eutherians, or placental mammals, and metatherians, or marsupials, such that therian mammals have at least one cysteine while egg-laying mammals’ k-casein contains no cysteine.8 This change in k-casein structure allowed dimerization, and even though mutations occurred since the ability to dimerize has been maintained, it must confer an evolutionary advantage. It is also important to note that in all species, cysteine is absent in the caseinomacropeptide (CMP) fragment (k-casein after Met106), suggesting that surface cross-linking disrupts micelle formation, promoting aggregation and losing its evolutionary advantage.6 The evolutionary diversity of k-casein led Manguy and Shields to conclude that “k-casein compositional and positional constraints appear to be influenced by modification preferences, protease evasion and protein-protein interactions”.6 Evolutionary adaptations in ten species saw large insertions of typically tandem duplicates, where all fragments are confined to the water-soluble fragment CMP.6 Included in these species are placental mammals, where large inserts are only observed in the CMP, while across species, the length of para-k-casein (PKC: k-casein before Phe105) remained unchanged. PKC length is constrained to preserve its role in stabilizing the internal micelle structure.6

AAs involved in phosphorylation or O-glycosylation were also influenced by evolutionary pressure in k-casein. Phosphorylation and O-glycosylation are possible on serine, while threonine only is glycosylated. In many species, the CMP fragment preferentially has threonine and not serine, while PKC greatly favors the inclusion of serine, suggesting preservation of the glycosylated hairy layer and the steric and electrostatic repulsion.6 The positive charge distribution is remarkably different between CMP and PKC; lysine is found through para-k-casein, while histidine and arginine are predominately in the CMP fragment.6 Both pepsin and trypsin cleave at arginine and lysine but not after histidine, which may give rise to selective pressure. Leucine and isoleucine are differentially placed on the PKC and CMP, where leucine is almost absent from CMP, while isoleucine is abundant.6 It was hypothesized that preferential placement of isoleucine in the CMP facilitates the early release of isoleucine over leucine and may provide a signaling advantage over leucine in metabolism-controlling response cells.6,9 Although species have different ratios and charged AAs; the CMP conserved mostly negative change with positive changes conserved near the chymosins cleavage site.6 Conservation of the charge distribution aligns with the electrostatic repulsion of the casein micelle hairy layer. PKC has maintained a greater density of chymosin cleavage sites (before and after aromatic AA and leucine), and with CMP’s preference for isoleucine over leucine, these suggest enzymatic cleavage favors removal of the casein micelle’s hairy layer and not complete digestion of the protein.6 This begs the question, what is the evolutionary advantage of renneting milk into cheese during neonate development?
Casein Cheeses
Rennet transforms milk into a strong and rubbery gel during curdling, and their aged cheeses are much more elastic (solid-like) than the fragile cheeses that are only acidic coagulated. Cheese curds are made by acidifying milk, denaturing the proteins, and using a starter culture containing one or more of Lactobacillus, Lactococcus, Propionibacter, or Streptococcus depending on the type of cheese made acidifies milk to the casein proteins isoelectric point (pH~4.6), which are then coagulated by renneting the milk (cleaving the Phe105-Met106 bond)using the proteolytic chymosin or rennet (the complex set of proteolytic enzymes of the calf stomach). Hydrolyisng this bond removes the stabilizing hairy layer and the exposed hydrophobic patches, causing the casein micelles to interact via hydrophobic attraction, which forms a continuous gel network.
Before genetically modified organisms (GMOs), rennet was traditionally obtained from the byproducts of the veal industry, where it was extracted from calves abomasum, which today accounts for <5% of industrially used rennet. The genes for chymosin produced by animals were inserted into bacteria, fungi, and yeasts, allowing them to produce recombinant chymosin during fermentation, which is now most commonly obtained from the fungus Aspergillus niger and the yeast Kluyveromyces lactis and accounts for most of the chymosin used in the cheese industry.
After the milk has been pasteurized, it is first curdled using a combination of starter culture and chymosin, and once it forms a wet rubbery gel, it is cut (into small cubes) and salted, allowing excess water, containing about half of the milk solid, to drain from the curd during wheying. The drained fluid contains the most soluble whey proteins often used for acid-set cheeses such as ricotta. After curdling, depending on the cheese variety, it may be stretched, alining the casein network and forming stringy cheeses such as mozzarella and provolone, while cheddaring stacks and presses the cheese curds, allowing its weight to cause more syneresis, creating drier, lower moisture cheddar, and finally they can be washed to remove excess salt and acid, producing milder varieties of Edam and Colby. Some cheeses require an additional ripening step where additional mold (typically but also bacteria) is applied to the surface of the cheese, allowing lipolysis and proteolysis, resulting in soft cheese (Brie and Camembert) and strong flavor development.
Whey Proteins

Unlike casein proteins, which are insoluble at pH 4.6, whey proteins are soluble and are obtained as a byproduct of cheese-making after curdling and draining. The primary whey proteins include beta-lactoglobulin (~55%), alpha-lactalbumin (~20%), Bovine serum albumin (BSA) (~6%), immunoglobulins (~10%) and glycomacropeptide (GMP) which is the hairy layer fragment of kappa-casein from amino acid 104-169 (12-20%). Other minor proteins include lactoferrin (2%) and lipoperoxidase (0.5%). Whey remaining after cheese making also contains lactose (~5%) and small amounts of lipids.
Some whey byproducts are converted to whey protein powder, which can be spray-dried or filtered to remove lipids and lactose before spray-drying into a glassy powder. Whey proteins do not aggregate upon renneting or acidifying milk; however, they are readily denatured by thermal processing, which unfolds the whey protein promoting hydrophobic interactions with other proteins, to form a protein gel. The left-over whey protein can be converted into cheese by concentrating the whey or using heat to coagulate the protein into a gel used to make Ricotta cheese. Ricotta is made by allowing the whey to ferment to become more acidic, making the proteins more prone to denaturation, causing the proteins to flocculate.
In addition to making cheese with whey, the proteins are functional ingredients used as emulsifiers in sauces and condiments, as thickeners for Greek yogurt, and as protein supplements. Protein supplements obtained solely from dying whey contains 30-90% protein and are termed whey protein concentrate (WPC), while whey protein isolates (WPI) are greater than >90% protein and require an additional membrane filtration or dialysis step to remove lactose and lipids. WPCs or WPIs can also be hydrolyzed to make protein supplements more digestible and water-soluble.
Works Cited
1 McSweeney, P. L. H. & Fox, P. F. (Springer, New York, NY, 2013).
2 Dalgleish, D. G. On the structural models of bovine casein micelles—review and possible improvements. Soft Matter 7, 2265-2272 (2011).
3 Meale, S. J., Chaucheyras-Durand, F., Berends, H., L.L., G. & Steele, M. A. From pre- to postweaning: Transformation of the young calf’s gastrointestinal tract. Journal of Dairy Science 100, 5984-5995 (2017).
4 Ahmed, A. F., Constable, P. D. & Misk, N. A. Effect of orally administered cimetidine and ranitidine on abomasal luminal pH in clinically normal milk-fed calves. American Journal of Veterinary Research 62, 1531-1538, doi:10.2460/ajvr.2001.62.1531 (2001).
5 Kageyama, T. Pepsinogens, progastricsins, and prochymosins: structure, function, evolution, and development. Cell Mol Life Sci 59, 288-306, doi:10.1007/s00018-002-8423-9 (2002).
6 Manguy, J. & Shields, D. C. Implications of kappa-casein evolutionary diversity for the self-assembly and aggregation of casein micelles. Royal Science Open Science 6, 190939 (2019).
7 Palmer, D. S. et al. Bovine chymosin: a computational study of recognition and binding of bovine kappa-casein. 49 (2010).
8 Kawasaki, K., Lafont, A.-G. & Sire, J.-Y. The Evolution of Milk Casein Genes from Tooth Genes before the Origin of Mammals. Molecular Biology and Evolution 28, 2053-2061, doi:10.1093/molbev/msr020 (2011).
9 Zhang, S., Zeng, X., Ren, M., Mao, X. & Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: a review. J Anim Sci Biotechnol 8, 10-10, doi:10.1186/s40104-016-0139-z (2017).


