Amino Acids

Proteins are polymers of proteinogenic amino acids, meaning they are biosynthetically incorporated into proteins during translation and ideally account for 10-30% of the daily caloric intake. Amino acids are of utmost importance in providing the building blocks to construct proteins such as collagen, enzymes, and organelles, as well as enzymes to break down foods, grow, repair tissue, and other bodily functions. Amino acids share homology, where a central carbon is attached to an amino and carboxylic acid group, and variations of amino acids arise from the side group attached to the central carbon.
Amino acids share homology, where a central carbon is attached to an amino and acid group, and variations of amino acids arise from the side group attached to the central carbon. Amino acids are subdivided into essential, which the body cannot synthesize; nonessential, which the body synthesizes; and conditional, which are not usually essential but can be during stress or illness. Nonessential amino acids include alanine (ala), arginine (arg), asparagine (Asn), aspartic acid (Asp), cysteine (Cys), glutamic acid (Glu), glutamine (Gln), glycine (Gly), proline (pro), serine (Ser), and tyrosine (Tyr); of those arginine, cysteine, glutamine, tyrosine, glycine, proline, and serine are conditional amino acids; nine essential amino acids include histidine (His), isoleucine (Ile), leucine (Leu), lysine (Lys), methionine (Met), phenylalanine (Phe), threonine (Thr), tryptophan (Trp), and valine (Val).

All 9 must be present in a food protein to be considered a complete protein, otherwise food sources need to be combined to compensate each other’s deficiencies.


Naturally occurring L-amino acids are also categorized based on their side chain, polarity, and functionality. Side chains containing only carbon and hydrogen are aliphatic and subdivide if they are linear (Gly, Ala, Pro), branched (Val, Leu, Ile) or phenylic (aromatic (Phe)). Non-aliphatic amino acid side chains are either nitrogenous, sulphurous, or oxygenic. Sulphurous amino acids contain sulfur and include Cys or Met. Nitrogen-containing amino acids include Lys, Arg, and phenylics amino acids His and Trp; and amides Asn and Gln, which also contain oxygen and are thus considered oxygenic. A hydroxylic amino acid contains its oxygen in a hydroxy group and includes amino acids Ser, Thr and Tyr, the last of which is phenylic; while carboxylic acids are the final subset, including Asp and Glu.
Polar positively-charged amino acids at physiological pH (~7.4) include arginine (Arg, R), histidine (His, H), and lysine (Lys, K); polar negatively-charged amino acids include aspartic acid (Asp, D) and glutamic acid (Glu, E). The polar uncharged amino acids are serine (Ser, S), threonine (Thr, T), asparagine (Asn, N), and glutamine (Gln, Q); hydrophobic side chains include alanine (Ala, A), valine (Val, V), isoleucine (Ile, I), leucine (Leu, L), and methionine (Met, M), phenylalanine (Phe, F), tyrosine (Tyr, Y), and tryptophan (Trp, W). Glycine (Gly, G), proline (Pro, P) and cysteine (Cys, C) comprise the last category of special amino acids.

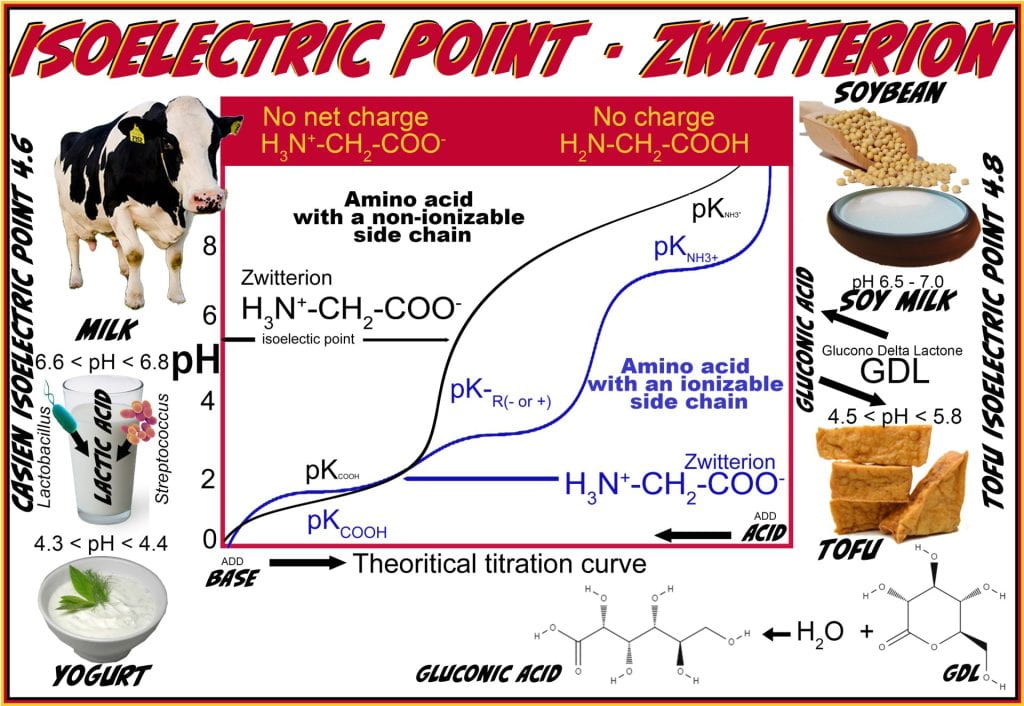
Isoelectric point and Zwitterions
The amino, carboxylic acid, and some side chains (Asp, Glu, Lys, Arg, and His) on amino acids are ionizable, each with an acid dissociation constant (ka), which measures how complete the acid dissociation is in solution. In other words, it measures the strength of acid by how tightly a Bronsted acid holds the proton. Large Ka corresponds to stronger acids, which mostly dissociate into their ions, and lower pKa values (pKa = – logKa). While each ionizable functional group has a pKa, molecules are characterized by their isoelectric point (PI). The isoelectric point is defined by the pH at which the net charge of a molecule is zero. The molecule is a zwitterion at the isoelectric point, meaning it contains a separate positive and negative charge:
NH3+-C(R)-COO–
For molecules without ionizable side chains, the pI is the average of:
pI = ½ (pKa1 + pka2)
where pKa1 is for the α-carboxyl group, and pKa2 is the α-ammonium ion. In the case of acidic side chains (Asp, Glu), the pI is at a lower pH because the acidic side chain introduces an additional negative charge; therefore, the neutral form occurs under more acidic conditions needed to neutralize the negative charge. Considering the dissociation constant for the amino acid side chain (pKa3), in the case of an acidic amino acid where the pI is at a more acidic pH thus:
pI = ½( pKa1+ pka3)
Conversely, amino acids with basic side chains (Lys, Arg, His) have a higher pI because the basic side chain introduces an additional positive charge; therefore, the neutral form exists under more basic conditions:
pI = ½( pKa2 + pka3)
For proteins, the isoelectric point cannot be predicted solely based on the sum of amino acids; it is typically experimentally determined using an electric field; if the protein does not migrate, then it is at the pI, and the protein is a Zwitterion.
Amino Acid Hydrophobicity

Amino acid solubility varies greatly depending on the side chain; as water or ethanol solubility decreases, they increase in hexane or oil solubility. Due to the ionization of amino acids at different pH values, their solubility in water (hydrophilicity) increases as the water interacts more with ionized functional groups than polar groups. Amino acids that contain large aliphatic (CH) groups (Ile, Leu, Phe, Trp, Val) tend to be poorly soluble in water and are hydrophobic, and since these side chains do not ionize, they are less affected by pH than ionizable side chains (Glu, His).
Positivity-charged amino acids are more soluble at low pH, while negatively charged (Glu, Asp) amino acids are more soluble at near-neutral pH. Few non-ionizable amino acids are hydrophilic and include Pro, due to its cyclic ring, and Gln, which only contains hydrogen on its side chain. When amino acids polymerize into proteins, the change in the protein solubility is not the sum of the amino acid hydrophobicity, which does not correlate to a protein’s overall solubility. This lack of correlation is because a protein polymer is highly flexible and can fold in such a way as to shield long sequences of hydrophobic amino acids from water by arranging the segments containing numerous hydrophilic amino acids at the surface.


