Refining of Sucrose From Sugarcane and Sugar Beets
Simple sugars, including mono and disaccharides, are found in many whole foods; however, there are limited sources to obtain purified ingredients through extraction and refining. The major sources of commercial refined sugars include sucrose from sugarcane and sugar beet, lactose valorized from dairy waste streams, and glucose, fructose and maltose refined from corn and cereals. Syrups such as maple, agave and honey contain more water than crystalline sugar and a greater diversity of sugars. Honey contains glucose and fructose, while maple syrup contains sucrose, glucose, fructose and complexes thereof, manganese, zinc, magnesium, calcium and potassium, vitamins and polyphenols.
The vast majority of sucrose is supplied by sugarcane plantations producing approximately two billion tons annually, while sugar beet account for ¼ billion tons and is processed using similar methods. Raw products are crushed, causing cellular decompartmentalization to facilitate separating fluids (70% total mass) from the remaining bagasse (~30% total mass). Solids in the sap following crushing are filtered out, and the remaining sap is boiled to increase the concentration of sugars, and some impurities are skimmed off the surface as they form a froth. The bagasse is often used as the fuel to boil the sap. Granular sugar is added after boiling and cooling the solution, seeding crystal growth and allowing the supersaturated sugars to precipitate onto the added sucrose crystals alongside many impurities. Cane sugar has many names depending on location rapadura (Brazil), jaggary (India), panela (Latin America), and kokuto (Japan) and also non-centrifuged cane sugar, as this is the first major delineation between other processed sugars.
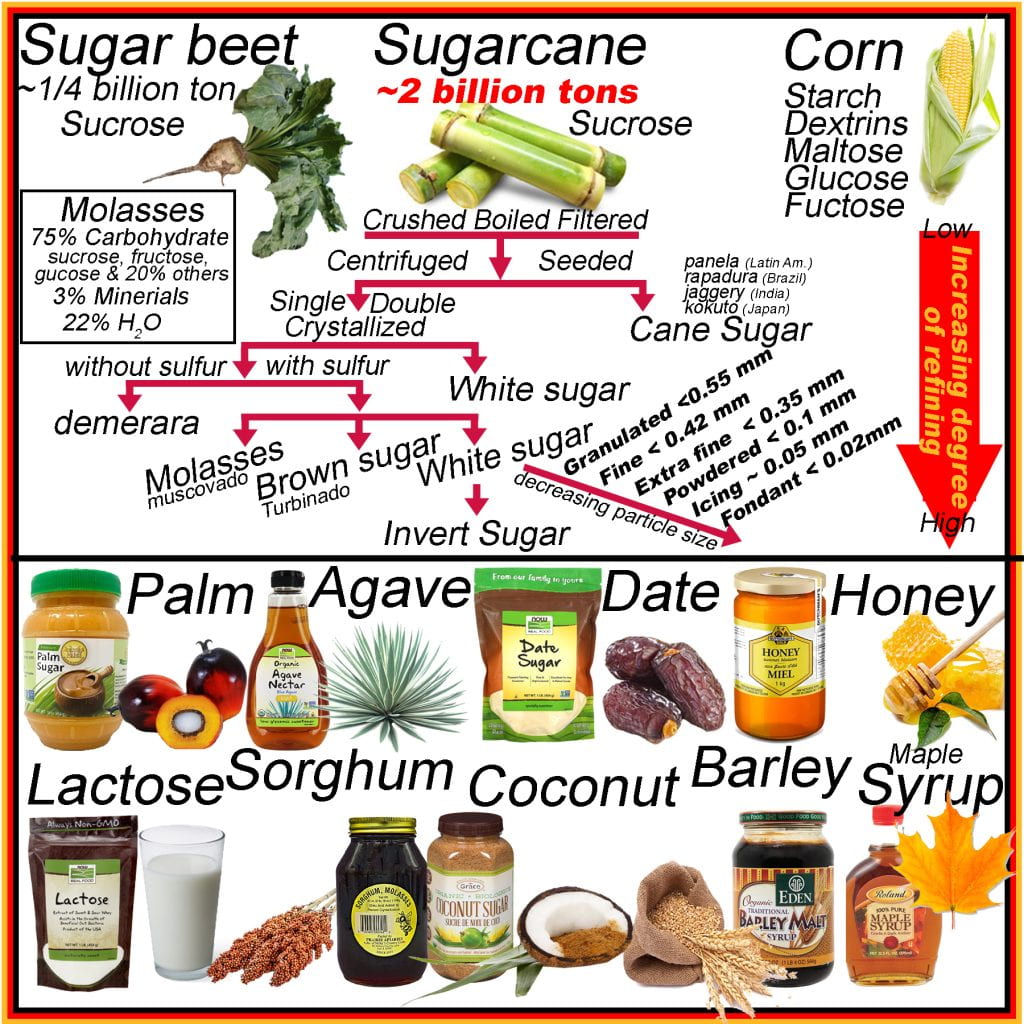
Instead of seeding to produce cane sugar, the cooled solution is refined by centrifugation with or without adding organic sulfur to prevent off flavors from forming. Without adding organic sulfur, demerara is produced, which has a bitter flavor. Ensuing centrifugation, crystallization and a second stage of centrifugation, pure crystalline sucrose (white sugar) is obtained by separating the molasses from the sucrose in cane sugar. While molasses, or muscovado, is primarily sugar, it also contains minerals, water and other compounds, making it appear dark brown to black. Brown sugar or turbinado contains both molasses and sucrose.
The granular white sugar (~0.5 mm) is pulverized to different extents depending on the application. White sugar is commonly available, in order of decreasing particle size, as fine (<0.42 mm), extra or ultra-fine (< 0.35 mm), powdered (< 0.1 mm), icing (0.05 mm) and fondant (0.02 mm). Sucrose or white sugar may be treated with invertase to obtain glucose and fructose (or invert sugar). Invert sugars are termed so due to the optical rotation of light for sucrose compared to glucose plus fructose is the opposite.
Refining of Starch
Carbohydrates from corn, cereal and potato are high in the polysaccharide starch and undergo different processing steps to acquire the starch granules. For corn, once cleaned and de-husked, the kernels are steeped at ~ 50oC in slightly acidic water, allowing water to absorb, increasing the moisture content from 15 to 45% and doubling the kernel volume. The acidic water weakens gluten bonds causing the starch to release, after which the corn is coarsely ground, breaking away the germ layer. Cyclone separators remove the low-density (~ 85% of the oil) germ, which is washed and screened to ensure all starch is removed, then the germ layer is further refined into corn oil. The remaining corn and water slurry is impact-milled, a much more vigorous grinding than previous, to release the starch and gluten from the fiber in the kernel, which is then screened and washed out, leaving a starch-gluten suspension or mill starch. The starch-gluten suspension undergoes centrifugation separating the lower-density gluten protein from starch. At this stage, the starch slurry contains < 2% protein, which is repeatedly washed and separated using centrifugal separation, leaving >99% starch after drying, and the product produced is unmodified corn starch.

Cleaned potatoes or tubers are rasped (shredded) with sulfur dioxide to prevent enzymatic browning, and the mash is sieved to remove fiber. In the mash fluid, starch granules are liberated into the fluid that also contains phenols and proteins, which in the presence of oxygen, undergo undesirable non-enzymatic browning reactions prevented by sulfur dioxide addition. The mash is repeatedly washed in aqueous sulfuric acid, and the starch fraction (starch milk (60% water)) is separated using centrifugation (cyclones). The starch milk is dried to a moisture content below 20% producing starch granules that are 50 to 100 nm and trace calcium, protein, potassium and iron.
Wheat milling today varies from coarse-ground whole flour produced by a single-pass rotating stone mill to much more advanced refining that purifies only the starch granules containing amylose and amylopectin. Advanced milling undergoes successive gap reduction between rollers; the first roller breaks the bran layer from the endosperm from the germ layers. The powder is sieved, removing fiber before the next size reduction by passing it through the successive rollers with a narrower gap. The subsequent powder is sieved with a smaller sieve pore size that removes the germ layer, and flour (90% starch & 10% protein) remains.

Modifications to Starch
Starches are modified with chemical, thermal and nonthermal or physical processes. Starches are modified to different extents where the degree of substitution (DS) for modified starch is targeted within high (1.5–3), medium (0.2–1.5), and low (0.01–0.2). The DS alterstheir applications as thickeners and stabilizers. Starch hydrolysis decreases their viscosity when in solution; however, the shorter dextrins produced become increasingly soluble and provide body and texture to confectionary products. Etherification of starch is mainly done to inhibit retrogradation, typically using propylene oxide, which adds a hydroxypropyl group onto starch. The introduction of a hydroxypropyl group alters polymer alignment leading to opaque gels. Esterification of starch modifies starch via acetylation, phosphorylation and succinylation. Monostarch phosphate (E1410) is added to products to prevent starch retrogradation. Phosphate distarch phosphate (E1413) is a chemically modified starch that replaces interchain hydrogen bonds with stronger permanent, covalent bonds between phosphates by treating starch with phosphoric chloride (POCl3). E1413 thickens soups, sauces and gravies and limits ice crystal growth during the freeze-thaw cycle. Succinylation of starches increases resistance to digestion while the succinyl group weakens the inter-molecular bonding facilitating swelling, solubilization and lower temperature gelatinization. Cationic starch varies in substitution from a low degree (< 0.07) to a high degree of substitution (≥0.07); a low substitution starch is positively charged and attracted to the negatively charged cellulose fiber and fillers resulting in increased fiber-to-fiber and fiber-to-filler bond strength important for papermaking. These starch also have applications in textiles and cosmetics, acting as thickening agents and emulsion stabilizers.
Crosslinking introduces new inter- and intra-molecular bonds on starch molecules in the granule, strengthening and stabilizing the granule. The benefit conferred to the starch is increased resistance to high or low temperatures and pH, maintaining the starch granule during processing since crosslinking decreases granule rupture and, consequently, loss of viscosity upon paste formation during cooking. These starches are commonly used in canned foods such as soups. Crosslinked compared to unmodified starches have decreased swelling capacity and solubility; however, resistance to retrogradation increases. Oxidation of starches converts hydroxyl groups to carbonyls (< 1.1%), which are either aldehydes or ketones, depending on their location, which impedes retrogradation. Oxidation of whole granules allows the granule to dissolve rather than swell and thicken, making them less susceptible to acidic environments. Succinylation with succinic acid anhydride increases paste viscosity and emulsion stability, lowers gelatinization temperature, and reduces glycemic response. It is used to stabilize beverages, mayonnaises, and battered meat.

Thermal treatments vary in temperature, presence of water, and annealing time, which alter starch structure differently. A key element of hydrothermal treatment is that the temperature must be above the glass transition but below the gelatinization temperature to maintain the starch granule size, shape and birefringence. Nonthermal modifications include shear (mechanical), pH, pressure ultrasonication and annealing (time)) which case structural changes to the granules, crystallinity and molecular structure, thereby decreasing digestibility and improving thermal and hydration stability and pasting characteristics. Both techniques tend to decrease digestibility and the basis for instant starches.
Starch Saccharification
Starch is suspended in water, heated to form a paste, and treated with amylase, which cleaves along the polysaccharides producing shorter dextrins by hydrolyzing the alpha (1 to 4) glycosidic bonds as the enzyme hydrolyzes glycosidic bonds, the hydrodynamic radius of the polysaccharide decreases, causing a decrease in solution viscosity. Recall that each polysaccharide in starch has a single reducing sugar, the first glucose in amylose or amylopectin; however, each time the polymer is hydrolyzed, a new reducing sugar at the end of the dextrin is created. Hence, colorimetric assays based on the Bendicts test are useful for monitoring the degree of hydrolysis. Additionally, the increase in reducing sugars (at the first glucose of each dextrin), a measure of the degree of hydrolysis, is calculated as a Dextrose Equivalent (DE). DE is the amount of reducing sugars present in a sugar product, expressed as a percentage on a dry basis relative to dextrose and ranges from 0, for starch, to 100 for glucose. Glucose syrups > 55 DE are ‘high conversion’; 35 < DE < 55 are ‘regular conversion’; and below 20 DE are maltodextrins. A maltodextrin with a DE of 4 has an average molecular mass of 3600 g mol-1; a DE of 10, the molecular mass decreases to 1800 g mol-1; and a DE of 20, the molecular mass decreases to 900 g mol-1. To illustrate the decreasing phase transition temperature, the glass transition temperature (Tg) for DE 4 is -5oC, which decreases to -11oC and -16oC for DE 10 and 20, while DE100, glucose, is below -40oC.

Maltodextrins, 3 < DE < 20, decrease in viscosity and increase in solubility as DE increases, but the polymers are still too long to be sensed as sweet. When the desired DE is achieved, the solution is spray-dried into a fine powder for maltodextrin. Maltodextrins are commonly used as bulking agents for pharmaceuticals and packets of sweeteners and as a thickener or filler to increase the volume of processed foods. DE > 20 are glucose syrups until DE 100, which is pure glucose and commonly used high DE glucose syrups include DE 42 – 55 are confectioners syrup. Glucose DE 42 is a powder, ~40% as sweet as sucrose, but the variation in polymer length impedes crystallization more than higher-DE glucose syrups and glucose. To obtain mono and disaccharides following alpha-amylase hydrolysis, pullulanase and isoamylase are added to cleave the alpha (1 to 6) glycosidic bonds. The resulting mixture of maltose and glucose can be separated and sold as either maltose or glucose or can be further treated with glucoamylase to obtain pure glucose. Throughout saccharification, the process is halted to obtain the desired mixture, and at any stage of hydrolysis, D-glucose ketoisomerase converts the glucose syrup to high fructose corn syrup and is done to increase the sweetness. While many of these products reach the processed food market, significant amounts of glucose are fermented by bacteria and yeast to produce alcohol, amino acids and other compounds.


