Solubility of Monosaccharide and Disaccharide
Recall that chalcogen atoms, or group VI hydrides, including oxygen (O), have lone electron pairs in their valence shells, causing these molecules to lack symmetry when covalently bound to two hydrogen atoms. All hydrogen chalcogenides, H2X, are polar triatomic molecules with a chalcogen, bent molecular configuration, and the presence of lone electron pairs that causes a deviation in the tetrahedral bond angle from 109.5 to 104.5 for water attaining the ability to act as a solvent able to solvate a solute. The bent structure and uneven charge distribution give rise to the dipole moment of water, which it uses to solvate the molecule by aligning its charge to both negative ions and polar molecules. Water is an excellent solvent, capable of solvating numerous charged and polar molecules. However, hydrophobic molecules such as vegetable oil do not form solutions and are discussed later under dispersions.

Because of its polarity, water forms electrostatic interactions with other polar molecules, and solutions contain two or more types of molecules that, when combined, are homogenously (evenly) distributed across the system. Each mono- and disaccharide in solution, at low concentrations, only interacts with water molecules and cannot form interactions with other solutes resulting in aggregation and often precipitation. Electrostatic interactions between water and the solute occur because water aligns itself according to the polar groups (-OH, -O-, and =O) on the solute charge (d– of oxygen aligns with positively-charged hydrogen on a hydroxyl group while the d+ of water’s hydrogens aligns with negatively charged oxygen or the acetal).
At low solute concentrations, there is a greater abundance of water than solute; when this is the case, water forms a hydration shell around the solute forming a clear solution. When the attractive forces holding the solute particles together are weaker than their water attraction, solvation occurs when water molecules completely hydrate the surface, forming a hydration shell. The hydration shell containing a solute molecule can diffuse readily, keeping apart by Brownian motion. Solvent molecules exceed solute molecules in a solution. All solvent-solute combinations have a solute concentration limit to their solubility; solubility is also highly sensitive to temperature, where increasing temperature increases the solubility threshold allowing more solute to be dissolved. Solutions remain clear with no particles larger than the diffraction limit of light.
Colligative Properties and Osmolarity
Colligative properties depend on the number of Osmoles (OsM), which is defined by osmoles of solute per liter where an osmole is one mole of dissolved and dissociated substance in water; hence 1 mole of disaccharide corresponds to 1 OsM while 1 mole of monosaccharide is still 1 OsM. If one mole of sucrose is treated with invertase, it hydrolyzes the glycosidic bond releasing a mol of glucose and a mol of fructose; thus, the osmolarity doubles as the mols increase from one to two. Colligative properties do not depend on molecular size or charge and are only affected by the concentration of dissolved solutes and ions. Solution colligative properties depend on the concentration of solute molecules but not on the identity of the solute.

Vapor Pressure and Water Activity
When a liquid is contained in a closed system (e.g., a sealed container impermeable to gas) with an air headspace, a fraction of molecules will escape the liquid phase evaporating into the gas phase of the headspace. Molecules constantly exchange between the phase liquid-vapor phases establishing an equilibrium vapor pressure above the liquid. Equilibrium vapor pressure is temperature dependent and affected by the cohesive attractive forces holding liquid molecules together. Water has strong cohesion due to strong intermolecular hydrogen bonds. High cohesion results in stronger attractive forces in liquids requiring greater kinetic energy to escape the liquid phase lowering the vapor pressure. Adding carbohydrates to water establishes a new equilibrium vapor pressure for the ‘solution’ or food that depends on the fugacity (f) or the ability to move into the vapor headspace. The addition of sugar at low concentrations, >~10%, follows Raoult’s Law:
“The partial pressure of each component of an ideal mixture of liquids is equal to the vapor pressure of the pure components multiplied by its mole fraction in the mixture.”
Water activity (aw), or the amount of water in equilibrium available to hydrate food components and a measure of the energy status of water in the system associated with deteriorative reactions, are derived from laws of thermodynamics. Water activity is the ratio of the fugacity (f) of water in food compared to the fugacity (fo) of pure water, and fugacity represents the tendency of water to escape from the system and closely relates to vapor pressure; hence water activity may be rewritten as: aw = p/p*

where is the vapor pressure established above the food and pi is the vapor pressure of pure water at the same temperature. Adding solutes and macromolecules to water decreases the entropy of the solution relative to pure water, resulting in a decreased vapor pressure, a colligative property. Materials have an aw between 1 and 0 (100% and 0% relative humidity) for pure and no water systems, respectively. Water activity negatively correlates with microbial growth, deleterious reactions, oxidations, and vitamin and color degradation. Since the added mono or disaccharide occupies space, effectively reducing the surface area of the air-water interface, where evaporation occurs, by the mol fraction of the solute and solvent. For carbohydrates, small molecules reduce water activity more than larger molecules. Carbohydrates listed from the greatest reduction in water activity to the lowest are: glycerol (triose), > tetraose > pentose (xylose) > hexose (5 member ring > 6 member ring) > disaccharide > hydrolyzed starch (Dextrose > Glucose syrups (Dextrose Equilivant (DE) (DE36 > DE25 > DE20 > DE10 > DE10) > maltodextrins > Dextrans) > starch. A low DE has undergone less hydrolysis than a high DE syrup; therefore, the high DE has smaller average molecular weights and is sweeter, having undergone more extensive processing.
Boiling Point Elevation
The boiling point of a liquid occurs at the temperature where the vapor pressure of the liquid is equal to the vapor pressure above. In an open system, at atmospheric pressure (1 atm), water boils at 100 oC. If the pressure above the liquid is reduced under vacuum, water boils at T< 100 oC, while water boils at T>100 oC if pressure is applied. Establishing why water boils at 100 oC is important to understand how the boiling point elevates when a solute such as salt or sugar is added. Added solute molecules occupy space, effectively reducing the surface area of the air-water interface, where evaporation occurs, by the mol fraction of the solute and solvent. This results in a lower partial pressure for the solution than pure water, which means there is a larger difference in the partial and atmospheric pressure and more energy or higher temperature is needed to boil a sugar or salt solution than pure water.
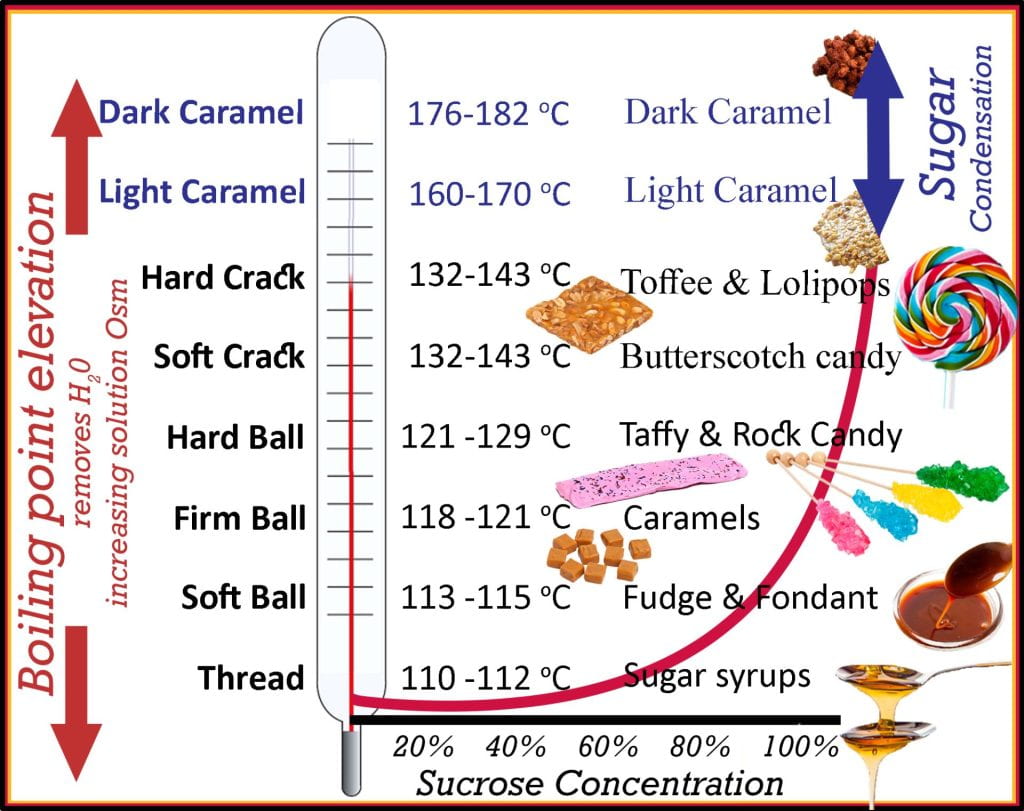
Boiling point elevation is essential in food science. Candy thermometers measure boiling point elevation, a useful parameter, perhaps most so in confectionery science, which is the science of sugar and fat. As a sugar water solution is boiled, water is removed as vapor, increasing the sugar concentration of the solution and osmolarity, establishing a higher boiling point. Based on Raoult’s Law, boiling point elevation determines the type of structure the solution will form upon cooling. At 110oC, the sugar solution is termed ‘thread,’ where the sugar concentration reaches ~85%. Threads are viscous fluids such as maple and corn syrups, and when the syrup is slowly added to cold water, it forms a liquid thread that will not ball up. At a boiling point of 113-115 oC, the ‘soft-ball’ stage increases in sugar concentration to 85% and forms fudges and fondants; when dropped in cold water, it forms a ball and flattens when removed. The ‘firm-ball‘ stage, at 87% sugar, boils between 118-121oC and is used to make caramels, forming a firm ball in cold water that retains its shape but remains malleable. The ‘hard-ball’ stage reaches 92% sugar and boils between 121-129oC, characterized by forming a hard-ball when the syrup is dropped into cold water; once removed, it will not flatten, but it remains soft enough to deform under pressure. The hard-ball stage is the endpoint for nougat, gummies, marshmallows and rock candy. The ‘soft-crack‘ stage increases sugar concentration to 95% with a boiling point between 132-143oC. Adding soft-crack to cold water solidifies into flexible, not brittle threads as they deform (bend) before failing (break). Soft-crack candies include saltwater taffy and butterscotch. ‘Hard-crack‘ candy, including toffee, brittle and lollipops, is boiled until 132-143oC, reaching 98-99% sugar concentration. When dropped into cold water, the sugar solution forms hard, brittle threads that are very brittle and not malleable. Beyond the hard-crack stage, at a temperature >160oC, boiling point elevation is no longer affected by the concentration as very little water is present (>99% sugar); at this point, sugar begins caramelizing, which is the condensation of sugar monomers into polymers of caramelans (C24H36O18), caramelens (C36H50O25), and caramelins (C125H188O80).
Carbohydrate Glass Transitions & Glassy Foods
To recap, the differentiation between crystalline (with a periodic arrangement of molecules across the crystal lattice) and glass (with amorphous solids with no regular periodicity) is the arrangement of molecules while both are solid. Glasses lack sharp diffraction patterns due to the amorphous structure and extremely low molecular mobility; thus, diffusion does not occur in glassy foods. The lack of diffusion indefinitely preserves them because deleterious reactions relying on diffusion do not occur. Enzymatic reactions are impeded because the enzyme and substrate cannot diffuse, preventing contact. Microorganisms cannot survive due to a lack of nutrients and waste product diffusion.

Glasses are extremely relevant in food science and include dried pasta, egg shells, spay dried milk and protein powders, instant coffee, hard transparent candies and dipping dots. In foods, crystallization during freezing often precedes glass formation because removing water as ice crystals increases the solute concentration in the unfrozen phase before the glass transition is reached. It is important to differentiate what molecules are involved in glass formation and which are the plasticizing molecules; in simple systems (e.g., mono- or disaccharide/water and polysaccharide/water). The plasticizing molecule (water) has a lower molecular weight than a glass-forming molecule (sucrose or polysaccharide), and it increases the molecular mobility of the system (adding more water makes it less viscous) and lowers the glass transition requiring colder storage. Even though sucrose is the glass-forming molecule in a sucrose/water solution, in a polysaccharide/sucrose/water system, sucrose and water will plasticize the glass-forming polysaccharide as both are lower in molecular weight. Glasses made from freezing foods start at the solubility limit and are cooled from T-1 to T-2, after which ice forms. During food freezing, water transitions from liquid to ice via nucleation and crystal growth and water is removed from the unfrozen phase as it forms ice (T-1 to T-2 on the graph), and the unfrozen phase, in effect, becomes more concentrated with solutes.

As the temperature continues to lower (following the phase boundary to T-3), further crystallization and more ice forms, continually establishing new equilibriums between the concentration of solute in the unfrozen phase and the freezing point depression. Cooling and ice formation continue until the eutectic point (T-3), and the solute sucrose should crystallize. However, crystallization is impeded as the sugar concentration increases drastically, coinciding with an enormous increase in the viscosity of the unfrozen phase (T4 and beyond). Beyond the eutectic point, freeze concentration continues until the glass transition is reached, causing a drastic reduction in molecular mobility. In this case, if more plasticizing molecule (e.g., water) is added, the glass transition decreases to colder temperatures.
If the temperature is not maintained below the glass transition, it becomes soft and sticky; in the case of dipping dots, the perfect spheres clump together. This state is often termed rubbery as it begins to soften and molecular mobility returns. Although the focus has been on glasses produced with small solutes, glass is also formed with polymers, typically called vitrification. Polymers, such as starch or gelatine, undergo vitrification (glass transition), after which they are completely immobile, producing a very brittle final product (dried pasta noodles). Near the glass transition is the rubbery transition, which still has a high viscosity; however, the product becomes malleable and often sticky or tacky. Increasing the water content in these products at a constant temperature will significantly reduce the viscosity. Hence it is said that water acts as a plasticizer for polymer glasses.
Increasing the molecular weight of the glass-forming molecule increases the glass transition temperature. Therefore, glasses made from polysaccharides transform through the rubbery state by adding water and heat. Dried pasta will not hydrate in cold water, but heating to 100 oC in excess water reduces the glass transition temperature, allowing the pasta to hydrate, dropping the pasta’s viscosity until edible. Hydrolysis of starch into maltodextrins of various sizes decreases the glass transition temperature proportional to the degree of hydrolysis; therefore, maltodextrin of DE 5 is 3600 g mol-1, has a Tg 160 oC; while the DE 36, with an average molecular weight of 500 g mol-1, Tg decreases to 100 oC. Baking complex food systems obtain stability and structure by removing sufficient water to reach the glass transition of the polymer or solute. Crackers, cookies, milk powders and hard candies all obtain their structure by creating an edible glass. The simplest system is hard candies. Typically, a sucrose solution is boiled to remove water. The sugar solution continually concentrates during boiling until approximately 82% of the final weight is sucrose, sufficient to produce a sucrose glass when cooled to room temperature. The result is the same as the unfrozen phase glasses discussed above.
Hard candies seem like crystalline solids but lack periodicity and present no crystal lattices. One major problem hard candies encounter is that sugar attracts water to absorb since it is very hygroscopic. Upon absorbing atmospheric water, the concentration of sucrose decreases, and eventually, a state transition occurs from the glassy state to the rubbery state, which causes the product to become sticky. Plasticization into the rubbery is why candies stick together when placed into a dish. Crackers and milk powders are also glasses; however, in this case, they rely on creating a glass due to the presence of polymers, including proteins and long-chain carbohydrates, such as starches. Water is removed from these foods through baking or spray drying, and a glass of the macromolecule is produced through vitrification. Vitrification is why crackers are brittle. Again if water absorbs, it plasticizes the glass, becoming soft and malleable, making them far less desirable and more susceptible to microbial growth. When milk powders or instant coffee powders absorb water, they also become soft, sticky, and lose viscosity allowing the porous structure to collapse, making them much harder to re-solubilize.


