The Small Intestine
The small intestine is a narrow tube approximately 3x the body length, subdivided into the duodenum (< 25 cm), jejunum, 3/5 of the length, and the latter 2/5, the ileum, together account for ~90% of the digestion and absorption of nutrients. Although the duodenum is short, it is the primary site where the chyme is neutralized by bicarbonate secretions and digestive enzymes produced in the pancreas (amylase, protease zymogens, procolipase, and lipase) and bile produced in the liver and stored in the gallbladder which enter the duodenum via the pancreatic and common bile duct. During this process, peristalsis slowly propagates the meal through the jejunum and ileum, mixing and neutralizing the chyme to the optimal pH for pancreatic digestive enzymes-humans primary source. The inner surface of the small intestine greatly increases its surface area through the jejunum and ileum as the mucous membrane lining the intestinal walls is highly undulating and with microvilli protruding which are highly vacuolated. The intestinal lining protects the basal cells from digestive enzymes, released early via the pancreatic duct in the duodenum and the jejunum via Lieberkühn glands, by secreting a thick mucus lining produced in Brunner glands. The transition between the sections is not obvious, the ileum tends to be narrower and less well-supplied with blood vessels, and peristaltic movements are slower than in the jejunum. Microvilli impairment decreases the intestinal surface area, decreasing macro- and micronutrient absorption, resulting in micronutrient deficiencies. Wheat proteins, or gluten, cause partial villous atrophy in individuals with the HLA gene or Celiac disease.
Bile, Colipase, and Lipid Digestion
Lipid digestion in the small intestine requires pancreatic lipase, colipase and bile, a mixed dispersion of bile salts, charged electrolytes such as sodium and bicarbonate, bilirubin (bile pigments), cholesterol, and lipids. Bile secretions serve a dual purpose: to eliminate waste hemoglobin and excess cholesterol; and, to aid in lipid digestion, making cholesterol, fat and fat-soluble vitamins absorbable in the jejunum and ileum. Bile acid is produced in the liver from cholesterol through the enzyme cholesterol-7-𝛼-hydroxylase which adds two hydroxyl groups and bile acid and then conjugates with glycine or taurine (amino sulfonic acid), which carries negative charges at physiological pH.
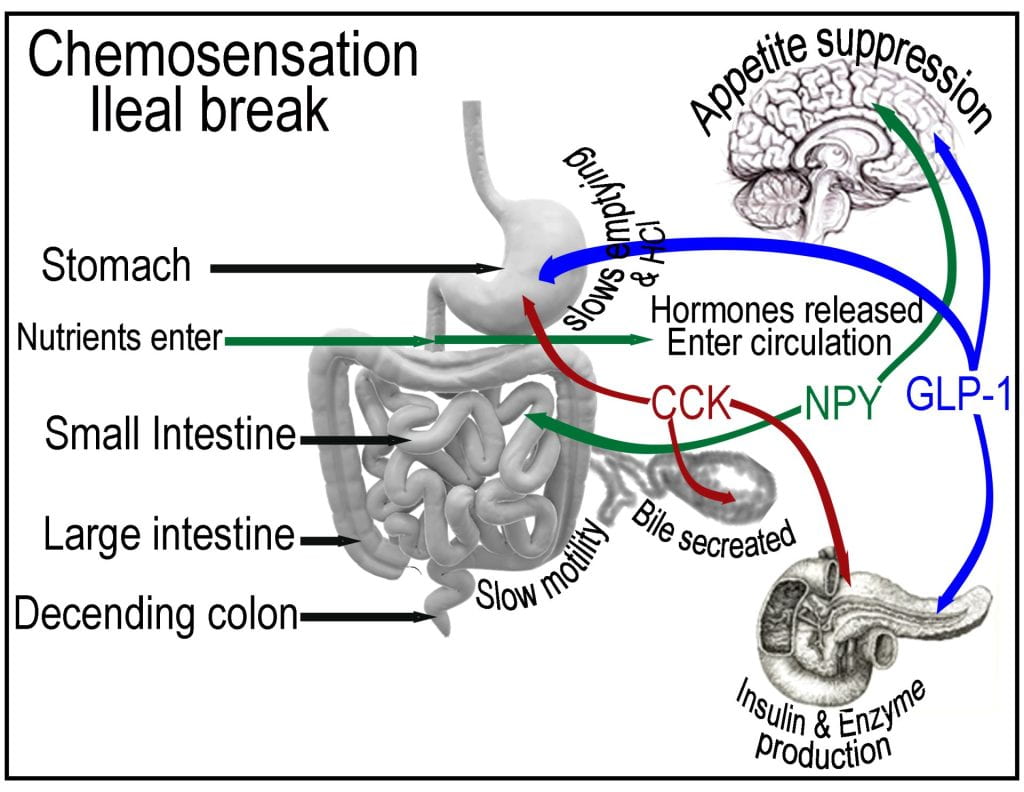
Post-prandial nutrient sensing in the small intestine triggers the ileal break, causing the release of cholecystokinin (CCK) and pancreatic polypeptide (PP) from the intestinal lining and pancreas, initiating negative feedback mechanisms that influence the function of the proximal gastrointestinal tract. In addition to suppressing appetite, CCK is also sensed by the gallbladder, causing the contraction and release of bile and on the pancreas stimulating the release of digestive enzymes. The gallbladder stores bile and senses the presence of cholecystokinin (CCK), which stimulates contraction, adding bile to the duodenum via the bile duct.
Unlike most surfactants, bile does not have polar and non-polar ends, instead one side of the molecule is hydrophobic, while the other side containing the hydroxyl groups is hydrophilic, allowing bile to emulsify fat into discrete droplets (< 50 µm), increasing the surface area for digestive enzymes to access. Bile maintains a negative change; thus, when it aggregates around a lipid droplet, it prevents fat droplets from coalescing via electrostatic charge repulsion. Bile interacts with fiber which can then be excreted or reabsorbed at the terminal ileum and returned to the liver via the portal system. Although pancreatic lipase adsorbs to the surface of triglyceride droplets, in the small intestine, droplets coated with bile salts, phospholipids and other surface active molecules prevent its access to triglycerides which only accounts for 5 mol% of the interface. The pancreas produces and secretes procolipase, a zymogen activated by the protease trypsin to colipase, preventing bile salts’ inhibitory effects on lipolysis of long-chain triglycerides. Colipase binds at the non-catalytic C-terminal of lipase and stabilizes an active conformation, increasing the binding site hydrophobicity. Procolipase has 95 amino acids and is hydrolyzed by trypsin at the Arg5-Gly6 peptide bond (Allouche et al., 2007). A 1:1 molar complex between lipase and colipase complex depends on the type of lipids being digested, altering lipase activity by the combined action of colipase and the lipid substrate (Erlanson-Albertsson, 1983). Colipase lacks enzymatic activity; however, it is essential for optimal lipase activity, as it anchors lipase onto the lipid droplet surface, providing access to the triglycerides buried in the droplet core and a surface composition of predominately (>95 mol%) surface-active molecules.
Pancreatic lipase contains the three amino acids (aspartate, histidine, and serine) catalytic triad that requires bile and colipase to emulsify the fat into discrete droplets and modify the droplet surface so the water-soluble enzyme can access the hydrophobic lipid substrates. Lipase is secreted in its active form and not as a zymogen because the absence of colipase prevents unintended lipolysis. Triglycerides contain three fatty acids esterified onto each of the three alcohol groups of glycerol. Pancreatic lipase preferentially cleaves fatty acids at SN1 and SN3 positions, resulting in the formation of an SN2-monoglyceride and two fatty acids that are absorbed using specialized lacteal vesicles, which are then assimilated and reassembled into TAGs and packaged into chylomicrons alongside cholesterol, phospholipid, 𝛽−Lipoprotein that enter lymphatic circulation until they reach the thoracic duct which drains into the bloodstream.

Pancreatic and Brush Border Enzymes for Carbohydrate Digestion
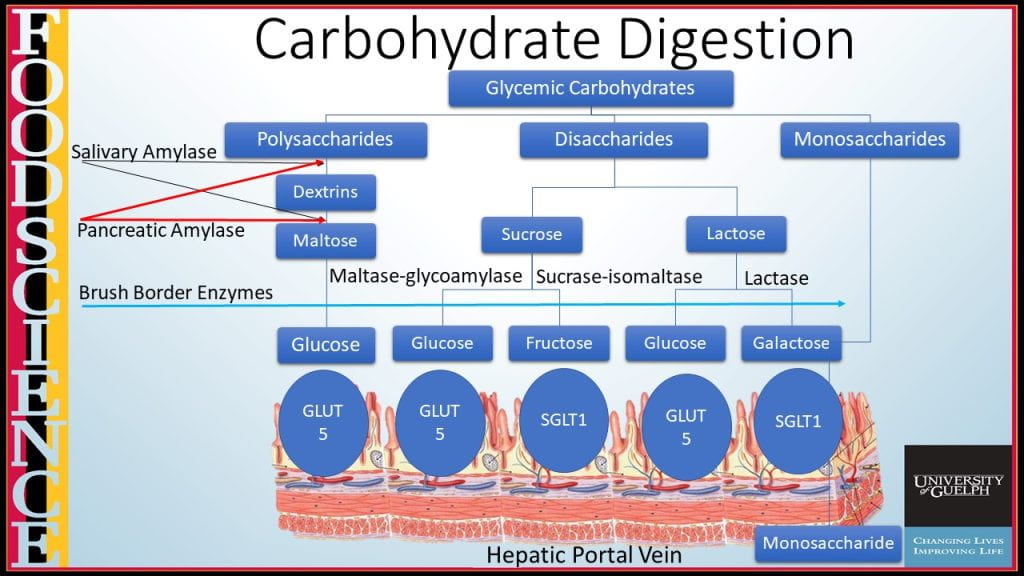
Carbohydrate digestion continues in the small intestine by pancreatic α-amylase, encoded by the AMY2 gene, an endohydrolytic enzyme that hydrolyzes α-1:4 glycosidic bonds in the middle of carbohydrate polymers, again only liberating short dextrins and no subunits small than the disaccharide maltose.
Terminal carbohydrate digestion of disaccharides such as sucrose, lactose, and maltose are hydrolyzed by brush border enzymes lactase (coverts lactose into glucose and galactose), maltase glucoamylase (converts maltose into glucose) and sucrase-isomaltase (cleaves sucrose into glucose and fructose, and converts isomaltose to glucose). Monosaccharides can then be transported across the microvilli from the luminal side either using passive facilitated diffusion via GLUT-5 transporter for fructose or facilitated diffusion requiring Na+ and ATP to transport glucose and galactose using the SGLT-1 transporter. On the capillary side, all three monosaccharides transport across by passive facilitated diffusion using GLUT-2.
Zymogens and Enzymes Involved in Protein Digestion
Protein digestion utilizes pancreatic proteolytic enzymes, which also secrete inactive zymogens trypsinogen and chymotrypsinogen to prevent self-digestion. The brush border enzyme, enterokinase, converts trypsinogen into the active form, trypsin, which then can modify chymotrypsinogen to chymotrypsin.

Both trypsin and chymotrypsin are endopeptidases fragmenting proteins into polypeptides; however, trypsin preferentially cleaves at amino acids lysine and arginine, while chymotrypsin cleaves at large hydrophobic residues tryptophan, tyrosine and phenylalanine. Terminal protein digestion utilizes exopeptidase (aminopeptidase, carboxypeptidase, and dipeptidase), which catalyzes the cleavage of the terminal peptide bond, releasing single amino acid, dipeptide or a tripeptide. Carboxypeptidase hydrolyzes peptide bonds at the carboxy-terminal (C-terminal) end, while aminopeptidases cleave at the N-terminus of proteins. Dipeptidases secrete from enterocytes of the small intestine to hydrolyze bound pairs of amino acids, dipeptides, into two amino acids before absorption.
Works Cited
Allouche, M. et al., 2007. Structure and Orientation of Pancreatic Colipase in a Lipid Environment: PM-IRRAS and Brewster Angle Microscopy Studies. Biochemistry. 46:15188-15197.
Erlanson-Albertsson, C. 1983. The interaction between pancreatic lipase and colipase: a protein-protein interaction regulated by a lipid. FEBS Letters. 162: 225-229.


