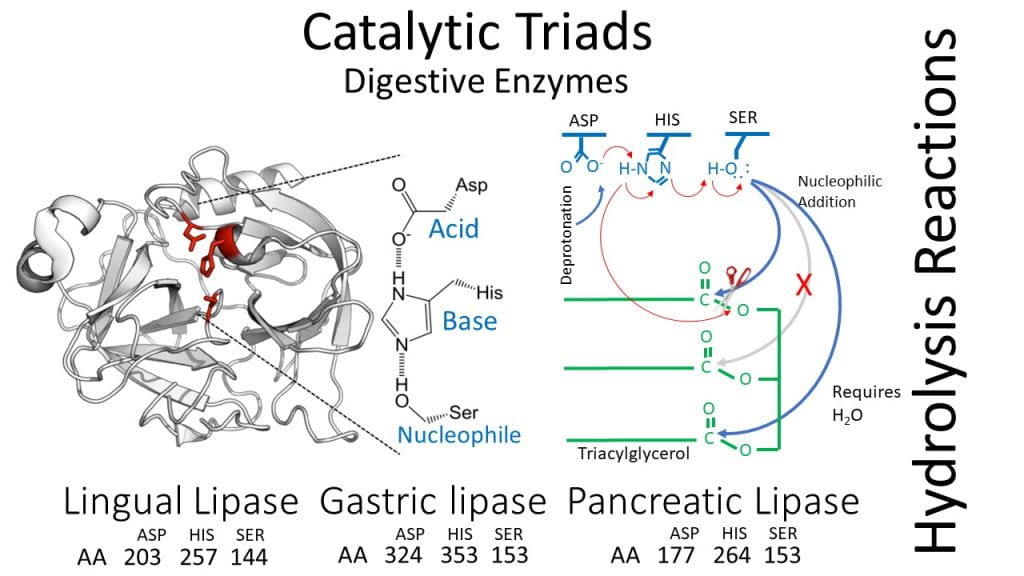
Catalytic triad
The catalytic triad is the most important functional element of digestive enzymes as it adds water during hydrolysis reactions breaking macromolecules into smaller functional units that can be transported across the epithelial layer. Digestive enzymes hydrolyze triglycerides into two fatty acids and one 2-monoglyceride, long-chain polysaccharides into dextrins, glucose, and maltose, and proteins into smaller peptides and amino acids. During digestion, hydrolysis reactions are catalyzed by enzymes containing the three amino acids catalytic triad with aspartate, histidine, and serine. Although all digestive enzymes contain these three amino acids, their location in the primary protein sequence differs, and their proximity to each other depends on the protein folding of their secondary and ternary structures. Aspartate for lingual lipase is at position 203, while for gastric lipase, it is at 324, and finally, for pancreatic lipase, it is at 177. Histidine for these three enzymes is located at 257 and 353 or 264. The location of serine is 144 for lingual lipase and 153 for both gastric and pancreatic lipases. The key to the catalytic triad is to overcome the fact that no amino acids are strong nucleophiles; hence the base in the catalytic triad polarises and deprotonates the nucleophile increasing its reactivity to form a covalent bond with the substrates’ carbonyl group.The amino acid aspartate in the catalytic triad operates by first deprotonating, initiating a charge relay sequence that polarizes and activates the nucleophile serine relying on the base histidine as an intermediate to relay charge. Once the nucleophile, serine, is activated, it forms an intermediate covalent bond with the substrate and, using water, hydrolyzes peptide, alpha-1-6 glycosidic, or ester bonds, depending on the enzyme.
Digestive Enzyme In the Oral Cavity

Two enzymes are secreted in the oral cavity as part of saliva, including lingual lipase and salivary amylase. Lipid hydrolysis is initiated in the oral cavity by lingual lipase, an acid-stable enzyme that does not require bile or co-lipase, and preferentially cleaves medium-chain triglycerides at the SN1 or SN3 positions, generating one free fatty acid and either 1,2- or, 2, 3-diacylglycerol. Lingual lipase relies on the aspartate, histidine, and serine catalytic triad located in the primary protein sequence at 203, 257, and 144. Human lipase, for the most part, cannot cleave SN2 fatty acids from triglycerides; the exception is maternal Iipase in breast milk. Lingual lipase remains active in the acidic gastric environment and small intestine; however, the proportion of lipid digestion completed by lingual lipase is low (~10%) in healthy adults and much more relevant to infant digestion. Also important for lipid digestion in the oral cavity is the incorporation of mucin, as it destabilizes oil-in-water acid-stable emulsions.
Energy and glucose are obtained from glycemic carbohydrates, including simple mono- and disaccharides, starch polymers (amylose, amylopectin), maltodextrins and glycogen, which undergo hydrolysis by a series of calcium metalloenzymes, the first of which is salivary amylase, an α-amylases produced in the oral cavity. Salivary amylase, secreted by salivary glands, is a single polypeptide, monomeric calcium-binding protein that hydrolyzes random α-1:4-glycosidic bonds along the starch chain but not α-1:6 bonds, which form the branch points of amylopectin. Salivary amylase is an endohydrolytic enzyme that hydrolyzes α-1:4 glycosidic bonds in the middle rather than at the end of the polymer, liberating short dextrins and maltose. Maltose is the smallest unit cleaved with α-amylase, and the concentration of salivary amylase produced in the oral cavity is proportionate to the copy variant number of the AMY1 gene, which was acted upon by the evolutionary pressure of a high starch diet. During mastication of high starch foods, a moderate increase in sweetness is perceived due to the action of α-amylase cleaving off free maltose units. In addition to its role in carbohydrate digestion, salivary amylase is also implicated in dental caries as it modulates the clearance and adherence of these streptococci to teeth. Salivary amylase has optimal activity near pH 6.7-7, at physiological temperature and requires chlorine ions. Once the chyme reaches the stomach, the high acidity and low pH inactivate salivary amylase.
Digestive Enzymes in the Stomach
2-3 L of gastric fluid is produced daily containing mucus, acid, intrinsic factor & enzymes. A thick mucose layer is secreted at the lining of the gastric compartment protecting the lining and musculature from the acidic environment and self-digestion. Entry of the bolus into the gastric compartment initiates the vagovagal reflex causing the release of acetylcholine, allowing further distension of the stomach to act as a reservoir for more food and to stimulate parietal cells to secrete hydrochloric acid. Vagus nerve impulses and satiety hormones gastrin and secretin stimulate the stomach to undergo acidification. Decreased pH is important in the stomach as it drops to 1.5, killing most bacteria, denaturing food proteins, causing them to unfold, and increasing their surface area for digestive enzymes to access. Chief cells located in the gastric pits of the stomach lining secrete the inactive zymogen pepsinogen and, when exposed to the hydrochloric acid excreted from the parietal cells, is converted to the active enzyme pepsin. In the case of proteases, to prevent self-digestion, inactive forms of the enzyme, zymogens, are produced that require an alteration in the structure before they can become catalytic enzymes. Unlike other human digestive enzymes, pepsin’s optimal activity occurs pH 1.5-2.0 compared to most other digestive enzymes with an optimal pH ~ 7. Pepsin is an endopeptidase that cleaves proteins in the middle liberating two smaller peptides but not individual amino acids. Gastric lipase is an acid-stable enzyme that does not require bile or co-lipase and preferentially cleaves short and long-chain triglycerides at the SN1 or SN3 positions, generating one free fatty acid and either 1,2- or 2, 3-diacylglycerol. Although not an enzyme, the intrinsic factor is a glycoprotein that binds vitamin B12, allowing its absorption at the terminal ileum.
Digestive Enzymes in the Small Intestine
After the gastric compartment, the bolus is converted to chyme, transits to the duodenum, and is mixed with pancreatic enzymes, bile, and co-lipase. Pancreatic enzymes include additional α-amylase, encoded by the AMY2 gene, lipase, two additional endopeptidases, trypsin and chymotrypsin, and nuclease (breaks down RNA and DNA). Pancreatic α-amylase is an endohydrolytic enzyme that hydrolyzes α-1:4 glycosidic bonds in the middle rather than at the end of the carbohydrate polymer, again only liberating short dextrins and maltose. Terminal carbohydrate digestion of disaccharides such as sucrose, lactose, and maltose are hydrolyzed by brush border enzymes lactase (coverts lactose into glucose and galactose), maltase glucoamylase (coverts maltose into glucose) and sucrase-isomaltase (cleaves sucrose into glucose and fructose, and converts isomaltose to glucose). Monosaccharides can then be transported across the microvilli from the luminal side either using passive facilitated diffusion via GLUT-5 transporter for fructose or facilitated diffusion requiring Na+ and ATP to transport glucose and galactose using the SGLT-1 transporter. On the capillary side, all three monosaccharides transport across by passive facilitated diffusion using GLUT-2.
The small intestine is the primary site for lipid digestion. Pancreatic lipase requires bile and co-lipase to emulsify the fat into discrete droplets and modify the droplet surface so the water-soluble enzyme can access the hydrophobic lipid substrates. Triglycerides contain three fatty acids esterified onto each of the three alcohol groups of glycerol. Pancreatic lipase only cleaves fatty acids at SN1 and SN3 positions, resulting in the formation of an SN2-monoglyceride and two fatty acids, which are assimilated and reassembled into TAGs that are then packaged into chylomicrons alongside cholesterol, phospholipid, 𝛽−Lipoprotein that enter lymphatic circulation until they reach the thoracic duct which drains into the bloodstream.

Pancreatic proteolytic enzymes also secrete inactive zymogens trypsinogen and chymotrypsinogen to prevent self-digestion. The brush border enzyme, enterokinase, converts trypsinogen into the active form, trypsin, which then can modify chymotrypsinogen to chymotrypsin. Both trypsin and chymotrypsin are endopeptidases fragmenting proteins into polypeptides; however, trypsin preferentially cleaves at amino acids lysine and arginine, while chymotrypsin cleaves the protein at large hydrophobic residues which as tryptophan, tyrosine and phenylalanine. Terminal protein digestion utilizes exopeptidase (aminopeptidase, carboxypeptidase, and dipeptidase), which catalyzes the cleavage of the terminal peptide bond, releasing single amino acid, dipeptide or a tripeptide. Carboxypeptidase hydrolyzes peptide bonds at the carboxy-terminal (C-terminal) end, while aminopeptidases cleave at the N-terminus of proteins. Dipeptidases secrete from enterocytes of the small intestine to hydrolyze bound pairs of amino acids, dipeptides, into two amino acids before absorption.
Digestive enzymes of the colon
While no endogenous digestive enzymes are produced in the large intestine encoded in the human genome, the microbiota contributes enzymes relevant to human nutrition as they can alter undigestable polysaccharides, polyphenols and B and K vitamins. Polysaccharides, especially soluble and non-soluble fiber and any residual starch, are fermented by saccharolytic bacteria producing short-chain fatty acid (SCFA) and other metabolites (lactate, succinate, etc.). Acetate (C2), propionate (C3), and butyrate (C4) are the most abundant SCFAs produced, and each plays a relevant role in human health, albeit complex, providing an energy source for the epithelial lining of the colon (colonocytes), altering gluconeogenesis, and potentially regulating appetite. These and other metabolites produced by saccharolytic bacteria cross-feed other bacteria; this interplay of availability of nutrients and waste products establishes a unique ecosystem in the ascending, descending, and transverse colons. Acetate production is common among commensal bacteria; Firmicutes tend to produce butyrate, while Bacteroides produce propionate. Depending on the host’s microbial population, gas production is variable during anaerobic fermentation as some species do not produce gas (lactobacilli and bifidobacteria). Proteins available in the colon that remain undigested, or contained in the mucin, or sloughed off the intestinal cell, or digestive enzymes are broken down into amino acids and shorter peptides by proteolytic bacteria to produce branched short-chain fatty acids (used as a biomarker) and gases H2, C02, H2S



