Biology of Taste and Aroma
Taste is stimulated when chemical compounds activate specialized receptor cells in the oral cavity and are essential to identify nutritious food items to assess if it is acceptable to consume as poor food selections when foraging wastes energy and increases potential exposure to harmful toxins if ingested (Breslin, 2013). Biological underpinnings controlling energy acquisition rely on complex relationships between taste, palatability, taste receptors, and hedonic responses to food and may provide answers as to why humans overeat beyond what is healthy (Calvo & Egan, 2015). Chemosensing and tasting molecules are inherited and provide genetic underpinnings to the sensory perception of foods. The best-known genetic polymorphism is the bitter-taste receptor TAS2R38 which discerns individuals who can sense phenylthiocarbamide (PTC) and 6-n-propylthiouracil (PROP). Bitter supertasters share the PROP phenotype and the same induced chemosensory experiences; for example, child PROP tasters have decreased liking and ultimately lower consumption of bitter foods (e.g., cruciferous vegetables) (Keller & Adise, 2016). PROP child non-tasters prefer and consume more fat and are prone to obesity, albeit confounding factors such as sex, food access, ethnicity, and socioeconomic status alter consumption and may require additional strategies to promote the consumption of bitter foods through the use of dips and sauces (Keller & Adise, 2016). Initial taste theory proposed that unique regions of the tongue were responsible for different sensations; however, it is now believed that taste is not site-specific and can be sensed on the outer and front of the tongue outside of the middle area. The ability to detect chemicals in the environment is manifested as aroma and taste; however, organs capable of sensing chemicals are not limited to gustation and olfaction, as most organs and the entire GI contain taste receptor cells capable of detecting compounds.
From a sensory perspective, the taste is perceived by the taste bud, a cluster of gustatory receptor cells in pores on the tongue papillae (circumvallate, fungiform and foliate). Four types of cells comprise taste buds, including Type I-III and precursor cells. Taste receptor cells are electrically active epithelial cells that depolarize and release neurotransmitters communicating with nearby neurons via synaptic transmission and intercellular communication using ATP and neurochemicals such as NPY, CCK, and Ghrelin. Compounds must first dissolve in saliva before they reach the papillae, where multiple taste cells are located. Type 1 cells provide the supporting structure and chemosensation receptors for salt, and type II contains receptors for sweet, umami (glutamate, mushrooms, meat and L-amino acids) and bitter and is the primary site for cognate hormone synthesis. Type III cells have conventional neuronal synapses with sensory afferent intragemmal nerve and are sensitive to acid chemicals perceived as sour. Taste precursor (Type IV) cells are small heterogeneous groups of cells at the base of the taste bud, which are quiescent precursor cells and immature taste cells that differentiate into Type I-III cells replacing the taste buds every 14 days. Perception of taste convolves the assessment of temperatures, tactile textures, pain sensations from the mouth, and aromatic compounds detected by the olfactory epithelium within the nasal cavity.
Each of the five tastes, sweet, salty, sour, bitter and umami, occur via different transduction mechanisms depending on the molecular composition. Salty perception is induced by sodium ions that directly excite taste neurons. Sour is perceived when acids bind to a thermoreceptor protein and increase the concentration of H+ ions, causing depolarization of the taste neuron. The remaining taste sensations, sweet, bitter, and umami, require G-protein coupled receptors (multiple for each sensation), exciting the specialized taste neurons. G-protein coupled receptors are not limited to the papillae and conscious quality assessments of taste as they are found in specialized enteroendocrine and tuft cells throughout the epithelial lining of the GI tract, constantly evaluating food composition during its passage through the GI, impacting gut motility (peristalsis) and secretions, appetite and peripheral nutrient utilization communicating through hormonal and neuronal signaling to the brain and periphery (Relmann, 2012).
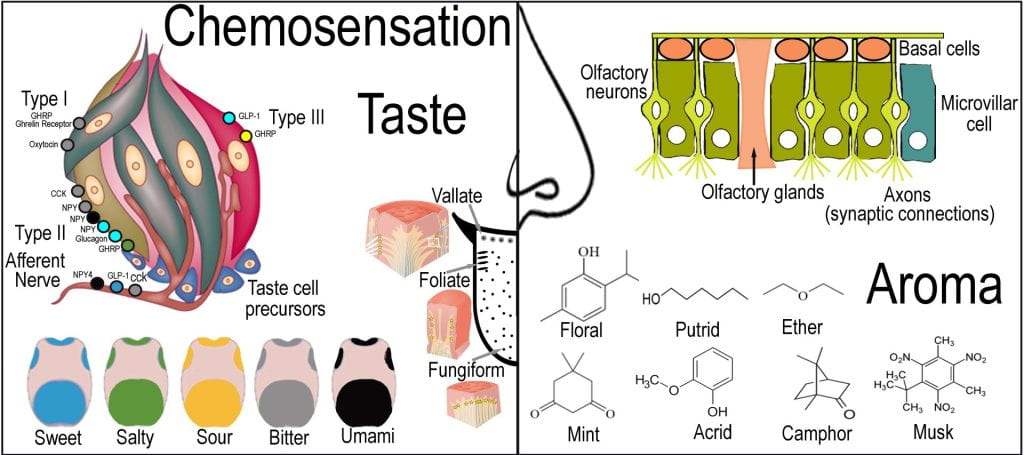
Receptors in the roof of the nose are localized to a small mucus membrane that detects small, airborne molecules as odorants using proteins found at the ends of axons in olfactory sensory cells that extend through pores of the underlying bone to the olfactory bulb. Neural signals for taste and aroma converge at the gustatory nerve, allowing flavor determination. There are seven primary sensations associated with aroma, each having different roles in food science. Short-chain alcohols such as butyric acid produce putrid perception and are important for avoiding rotting foods as they are breakdown products of protein and lipids. Flavors such as mint (spearmint, peppermint), ether (grapes, wintergreen, apples), and floral (sage, rosemary, orange) are extensively associated with foods, while camphor and acrid (smoked and cured meats) are less commonly used, and musk is not associated with the food industry. The structure of aromas includes alkanes, alkenes, alcohols, aldehydes, ketones, carboxylic acids, haloalkanes, thiols, amines, nitriles and lactones and the volatility inversely associates when the hydrocarbon chain length.

The precise mechanisms by which molecules stimulate taste and aroma receptors and how those signals converge, forming flavor and food preference, remain elusive. Two examples illustrate the importance of molecular structure on our perceptions of sweetness and aroma. The AH-B theory for sweetness (Shallenberger et al., 1969) links perception in molecules that have a bifunctional group consisting of an acidic (AH) and a basic (B) moiety with an A—H proton to B distance of about 3 Å. AH moieties for sweet molecules commonly include one of -OH, NH, -NH3+, while the common B moieties are -0-, =0, Cl, C-C=C-C, and COO–. Sweetness is perceived when molecules form two simultaneous H-bonds with AH and B on the receptor site and sweet compound.

Shallenberger also shows the importance of stereochemistry as L-enantiomers of monosaccharides are sweeter than D-enantiomers, while D-amino acids are sweet tasting and L-amino acids are tasteless or bitter. Sour perception is less well-established and proposed to use a similar mechanism as the AH/B theory, as the sour molecule contains a carboxylic acid (COOH) instead of the previously mentioned H-bonding groups. Cations initiate the sensation of salty, and the anion modifies its perception. Little is known about the mechanisms of binding for bitter and umami compounds. Olfactory neurons also distinguish isomeric differences for compounds. The terpenoid (CH2=C(CH3)−CH=CH2) carvone is found in caraway as the R-geometric isomer and in spearmint as the L-isomer. The aroma in caraway is highly aromatic, associated with anise and licorice notes, while l-carvone is used as spearmint.
Beyond the Five Basic tastes
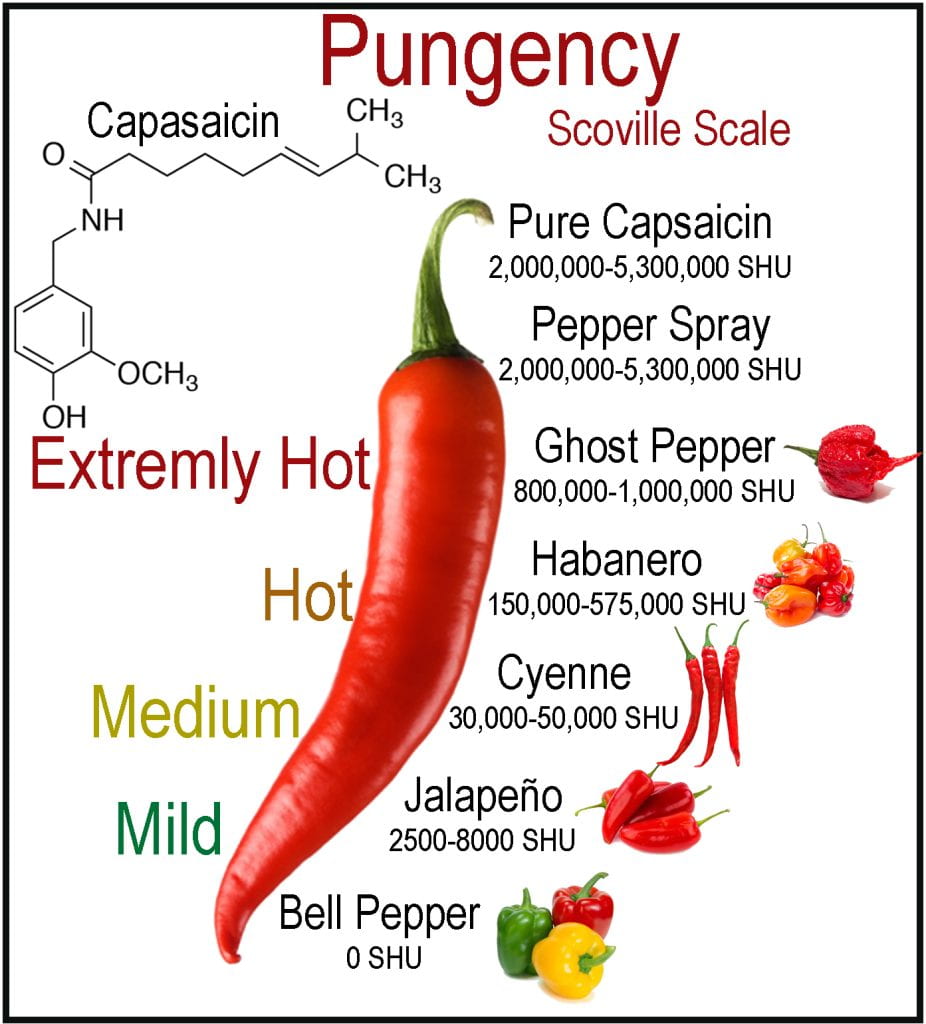
Beyond bitter, sweet, sour, umami, and salty other important oral sensations include pungency, astringency, and cooling. Pungency is the spicy sensation associated with Peppers. The degree of pungency is described using the Scoville scale, which measures the concentration of capsaicinoids, specifically capsaicin. Capsaicinoids are alkaloids that contain nitrogen and act as a base. The hydrocarbon chain on capsaicin decreases its solubility in water, which is why drinking water does not reduce the amount of heat perceived in the oral cavity and why milk is recommended, as the fat present can displace them from the tongue. Capsaicin is sensed by thermosensitive Vanilloid receptor-like type 1 (VRL-1) and cold menthol receptor type 1 (CMR1). VR1 activates above 41 °C, VRL-1 above 50 °C and CMR1 is activated between 7–28 °C.
Cooling sensations, caused most commonly by menthol, the most sold flavorant, also interact with cold receptors, CMR1, on the tongue, stimulating an increase in intracellular calcium concentration, which initiates the same nerve stimulus as cold water. The gustatory system contains different thermoresponsive neurons that contribute to taste (Cruz & Green, 2000). Astringency is caused by any compound that triggers constriction of tissue and is a dry puckering sensation in the oral cavity, often confused with bitterness caused by tannins. Tannins are polyphenols produced commonly in unripened fruits as a natural defense mechanism to prevent their consumption before seeds fully develop. Astringency is essential in red wines such as Merlots, tea, and coffee. The taste bud does not detect the oral sensation of astringency but instead is caused by tannins interacting and precipitating salivary proteins (e.g., amylase) onto the tongue surface. After mixing soluble tannins with mucin and proteins found in saliva, colloidal complexes become insoluble and precipitate on the tongue, causing a constriction detected through tongue mechanoreceptors.
Chemosensing and the Ileal Break
Chemo sensation is not limited to the oral and nasal cavities as chemosensing (taste cells) is found on nearly every organ. Direct evidence of chemosensation beyond taste and aroma is evident in utero through the discriminatory behavior of fetuses, which were seen to grimace after the mother consumed kale (bitter) and smile after carrot (sweet) consumption (Ustun et al., 2022). The gut is the largest hormone-producing organ, where gastrointestinal epithelial cells function as molecular chemosensors in multiple food intake and digestion processes. Interestingly, many of the hormones described in the gut-brain axis are also expressed in taste bud cells (Calvo & Egan, 2015). Satitey-inducing hormone receptors found on taste buds include receptors for GLP-1, CCK, NYP, PPY, Oxytocin, and ghrelin. The Ileal break (others define duodenum, jejunum, and ileum brakes) detects the entry and presence of nutrients (fat, carbohydrates, and/or proteins) into the duodenum and jejunum, providing negative feedback mechanisms altering the function of more proximal parts of GI tract (Maljarrs, et al., 2008). Nutrients entering the small intestine trigger the release of PYY and Glucagon-like peptide-1 (GLP-1) that act as signaling hormones that travel through the circulatory system. GLP-1 triggers a cascade of events as it reaches other organs stimulating satiation and suppression of appetite, slowing gastric emptying and acidification, and increasing insulin production in the pancreas to prepare for the influx of glucose, while PYY (Holzer, et al., 2012) alters intestinal motility, slowing the transit of chyme ensuring maximal nutrient digestion and absorption. Also suggested to be part of the ileal brake is the release of cholecystokinin (CCK) upon entry of lipids and proteins into the duodenum and jejunum (Lieverse, et al., 1995). The gastric compartment senses CCK and slow gastric emptying, while CCK triggers the gallbladder to contract, forcing bile into the duodenum. CCK acts on the pancreas to release digestive enzymes.

Works cited
Breslin, P.A.S. 2013. An evolutionary perspective on food and human taste. Current Biology. 23:R409-418.
Calvo, S.S.C. & Egan, J.M. 2015. The endocrinology of taste receptors. Nature Reviews Endocrinology. 11:213-2017.
Cruz, A., & Green, B.G., 2000. Thermal stimulation of taste. Nature. 403:889-892.
Holzer, P. et al., 2012. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut–brain axis. Neuropeptides. 46: 261-274.
Keller, K.L. & Adise, S. 2016. Variation in the Ability to Taste Bitter Thiourea Compounds: Implications for Food Acceptance, Dietary Intake, and Obesity Risk in Children. Annual Review of Nutrition. 36:157-182.
Lieverse R.J., et al., 1995. Satiety effects of the type A CCK receptor antagonist loxiglumide in lean and obese women. Biological Psychiatry. 37:331–5.
Maljaars, P.W.J. et al., 2008. Ileal brake: A sensible food target for appetite control. A review. Physiology & Behavior. 95:271-281.
Relmann, F., et al., 2012. G-Protein-Coupled Receptors in Intestinal Chemosensation. Cell Metabolism. 15: 421-431.
Shallenberger et al., 1969. Sweet Taste of D and L-Sugars and Amino-acids and the Steric Nature of their Chemo-receptor Site. Nature. 221: 555–556
Ustun, B. et al., 2022. Flavor Sensing in Utero and Emerging Discriminative Behaviors in the Human Fetus. Psychological Science. 33: 1651-1663.


