Removing Heat
Solid ice and liquid water are different phases with divergent physical properties. The difference between lattice structures alters molecular interactions in the liquid and solid states, meaning that the thawing and freezing of foods are two entirely different processes. The molecular order of ice increases the rate heat is transmitted, or its thermal conductivity, compared to unfrozen foods containing water. The thermal conductivity of ice is 2.20 W/m K and decreases to 0.56 W/m K when liquid. Additionally, the change in structure requires half of the heat to increase the temperature of ice, which has a heat capacity of 2.10 kJ/kg K, compared to liquid water (4.19 kJ/kg K). These two parameters and their densities (ice (916 kg/m3) & water (996 kg/m3)) are combined into a thermal diffusivity. The magnitude of the difference in thermal diffusivity leads to significantly longer thawing than freezing times. Freezing food in a sub-zero environment removes heat through the surface; hence, during freezing, ice propagates inward, and subsequent heat must be transferred across the frozen food.
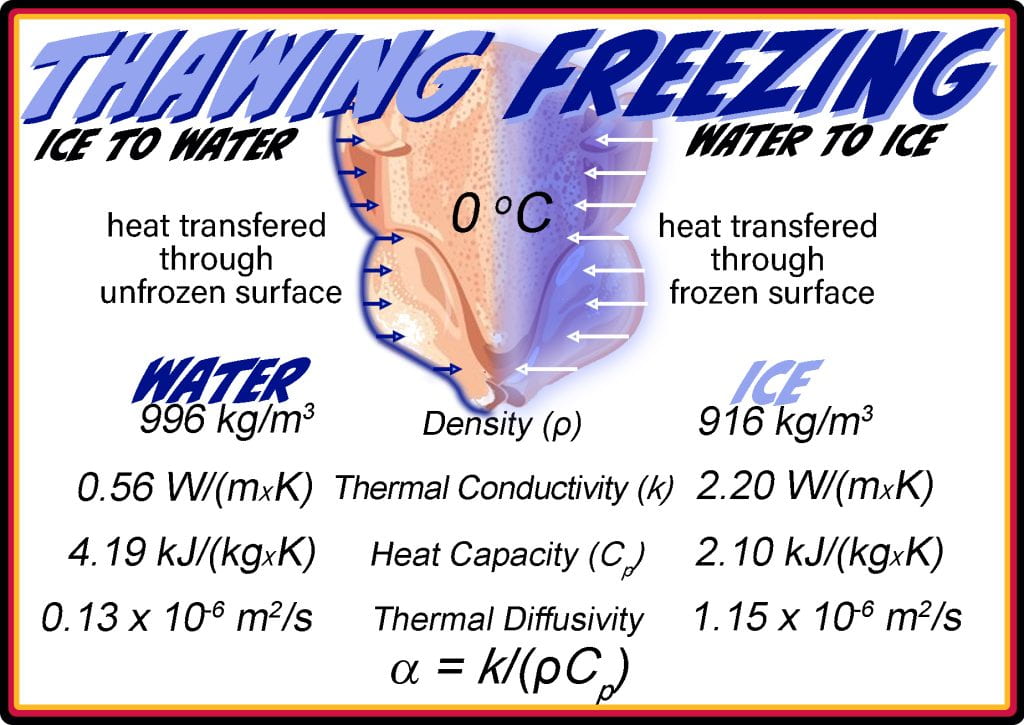
Conversely, the surface ice melts while thawing, and subsequent heat transfer occurs through unfrozen ‘liquid’ water in the food. Up to this point, only sensible heat, or the amount of energy causing a temperature change, is considered, not the latent heat. The latent heat of fusion, or the energy changed during a phase transition that does not lead to altered temperature, is the same when going from ice to water or water to ice.
Undercooling, Nucleation, & Crystal Growth
Differential Scanning Calorimetry (DSC) is the primary tool to determine melting and crystallization temperatures. A small amount of material is placed in a metal pan and then in the DSC alongside a blank (empty metal pan) on separate heat sinks composed of a different metal than the pan. Contact between two dissimilar metals produces an electrical current depending on temperature; thus, the contact between the pan and heat sink acts as a thermocouple where the thermal gradient between the blank and sample is monitored as current. Two different furnace configurations are commonly used in DSC; the heat flux device, depicted below, uses a single furnace, and the temperature between the samples is recorded, while for power compensation instruments, the sample and reference are heated by separate furnaces at the same rate, and the difference in power is monitored.

The heat flux between the sample and the blank remains constant until the transition temperature, after which the heat flux difference becomes larger due to the endo or exothermic nature of the transition. Crystallization is an exothermic process that releases heat while melting is an endothermic process that absorbs heat. Irrespective of how fast or slow the sample is heated, the melting temperature occurs at an exact temperature, in the case of pure water, at 0 oC and is the thermodynamic transition temperature. However, the same is not true for crystallization, which occurs at different, lower temperatures depending on the cooling rate, and the difference between melting and crystallization temperature is undercooling or supercooling.
Ice formation is a complex event involving undercooling and nucleation, followed by crystal growth. To explain undercooling, the thermodynamic properties of solids and melts, including their entropy (S) and energy (enthalpy (H)) as a function of temperature, has to be considered simultaneously. Entropy (ΔS) is the degree of disorder and randomness. Since crystallization is exothermic and spontaneous (ΔG < 0) (ΔG=ΔH-TΔS) by necessity, it produces entropy causing the temperature to increase. Therefore as the material is cooled and a few molecules aggregate to form a crystal embryo, they release energy upon formation at the crystal-liquid interface, which causes the unstable crystal embryos to dissociate quickly and melt.

For a crystal embryo to form stable nuclei able to facilitate further crystal growth, it must reach a critical size, after which the energy released by the formation of non-covalent interactions exceeds energy associated with the net entropic loss of the system (S > 0) incurred due to increased molecular order. Nucleation creates a new phase with a lower chemical potential than the current phase resulting in small clusters of water associating and orientating similar to ice. This small cluster of molecules or the crystal embryo is unstable through the early stages of nucleation due to excess free energy associated with creating the new surface/interface. Embryos and nuclei are spherical to reduce the surface area-to-volume ratio. Hence, the free energy of the embryo with radius r is a combination of surface (positive) and volume changes (negative):

where Aη is the surface area of the nuclei combined (with delta) the surface free energy per unit area, Vn is the nuclei volume, and ∆u is the difference in chemical potential between the solid and liquid and VmS is the solid molar volume. Nucleation occurs when the free energy associated with the phase change is negative; this does not indicate the reaction rate, just that the reaction will occur. Due to the free energy associated with a new phase, there is a critical size that the embryo must reach before it becomes stable. Small unstable nuclei have a high surface area to volume ratio; thus, the interfacial term (Aηd) is greater than the volume and chemical potential thus, ∆Gn is positive, and the system is thermodynamically unstable. The embryo must grow to a critical radius where the volume term exceeds the surface area term leading to negative free energy.
Primary nuclei appear from the melt, while secondary nuclei form in the presence of nuclei. Large degrees of supercooling lead to homogenous nucleation, where all nuclei appear simultaneously; conversely, low supercooling leads to fewer nuclei and often coincides with secondary nucleation, termed heterogeneous nucleation. Once stable nuclei form, crystal growth occurs spontaneously, and the crystal growth rate is limited either by the diffusion of molecules from the liquid state to the crystal surface (diffusion-limited crystallization) or the rate at which they are incorporated into the crystal lattice (reaction-limited crystallization). Partly crystalline foods contain numerous ice crystals suspended in a phase concentrated unfrozen phase, and as a result, there is a significant surface area between the two physical states of water. The large surface area and interfacial tension lead to thermodynamic instability causing the system to attempt to minimize its interfacial area causing small crystals to dissociate and large crystals to grow in a process termed annealing.
Post-Crystallization Annealing
In food science and nutrition, ice does not crystallize from pure water; instead, ice forms in either solutions or dispersions in the presence of other molecules. This obvious statement has two important consequences, the first, already discussed, is the colligative properties and freezing point depression caused by small soluble molecules. The other consequence is that the transition from water to ice in food systems is NOT a phase transition but a state transition. Phase transitions achieve endpoints in thermodynamic equilibrium and do not change as long as they are stored at constant temperature and pressure. Food, when frozen, contains a significant amount of unfrozen water, which has a huge surface area, complex interface and soluble and dispersed small molecules and biopolymers, leading to a non-equilibrium state.

The food system strives to reach equilibrium by minimizing the surface area between states via processes such as Ostwald’s ripening, causing ice crystals to grow during annealing. The tremendous amount of surface area is highlighted by the physical structure of ice cream, which has air-lipid and air-water interfaces, crystalline and liquid oil interfaces, and oil-water, ice-water and ice-lipid interfaces, all inducing instability in the product. Thus, to ensure product quality until the end of the shelf-life, kinetic barriers must be in place to slow the interface’s annealing or recrystallization and reduction. When the barriers are insufficient, separation and ice crystal growth cause detrimental changes altering consumer acceptance of the product. Recrystallization changes the number, shape and size of the ice crystals. Throughout storage, small crystals disappear and result in larger crystals growing to even larger crystals.
This type of recrystallization is Ostwald ripening, where large crystals grow, and small crystals disappear at a constant temperature and pressure. Temperature fluctuations accelerate recrystallization resulting in the depletion of small crystals and the increase in the size of larger crystals. The outcome results in grainy texture and consumer rejection of products. If ice crystals grow too large, they become sensible and induce a grainy texture; however, in whole foods, such as frozen fruits and vegetables and animal tissues, uncontrolled crystal growth disrupts endogenous barriers such as cell and organelle walls leading to decompartmentalization and drip loss, which is the transfer of water from myofibrils in meat or cells in plants to the extracellular space. Decompartmentalization allows substrates and enzymes to come into contact with what would otherwise be localized, causing deleterious reactions such as enzymatic browning, where polyphenol oxidase catalyzes the conversion of phenols to reactive quinones that form a brown pigment.
Cultivating plants in colder climates (e.g., Canada) need the plant to have a mechanism to ensure decompartmentalization does not occur. Plants have different tolerance to sub-zero temperatures depending on their ability to cryoprotect and limit the size and location of ice crystals. Some plants, including winter wheat, decrease moisture content upon cold acclimation; while others, such as sugar beets, produce monosaccharides such as raffinose. In both cases, solute concentration increases and the colligative properties they impart depress the freezing point. Some plants and cold-water fish have known mechanisms of cryoprotection that do not rely on colligative properties; for example, winter rye produces anti-freeze proteins. Although the exact mechanism of anti-freeze proteins is unknown, it is postulated that they bond to the facets on the ice surface, preventing new water molecules from adhering to the surface and thus preventing crystal growth and decompartmentalization.

Glass Transitions & Glassy Foods

Most solids are crystalline with a periodic arrangement of molecules across the crystal lattice. On the other hand, glasses are amorphous solids with no regular periodicity, and the molecules do not orient onto Bravais lattices meaning they orient in the same manner as liquids. X-ray diffraction of crystalline solids produces sharp peaks corresponding to reflections of molecules located on crystalline planes in the Bravais lattice, while glasses demonstrate no sharp peaks and only a broad amorphous scatter as there is no periodic spacing between molecules.
A glassy food is indefinitely preserved because there is no molecular mobility, and deleterious reactions relying on diffusion do not occur. Enzymatic reactions are impeded because the enzyme and substrate cannot diffuse, preventing contact. Glasses also lack ice crystals; therefore, there is no cellular disruption due to crystals rupturing plant and animal cells. Microorganisms cannot rely on the diffusion of nutrients and waste products. While it is theoretically possible to reach a glass transition of pure water by quench cooling to -134oC avoiding ice formation, from a food perspective, this does not occur because it will crystallize before the glass transition is reached. Also, since most foods contain numerous solutes and polymers, reaching a glass for any pure system is irrelevant to food science and nutrition. That said, glasses are extremely relevant in food science and include dried pasta, egg shells, spay dried milk and protein powders, instant coffee, hard transparent candies and dipping dots. Describing glasses of solutions and dispersion is slightly more complex than a pure system, but the same benefits are conferred, such as stability and impeded spoilage due to the extremely high viscosity making it behave as a solid. In a simple system such as sugar/water, polymer/water, or polymer/sugar/water, it is important to differentiate what molecules are involved in glass formation and which are the plasticizing molecules. A plasticizing molecule (water) has a lower molecular weight than a glass-forming molecule (sucrose), and it increases the molecular mobility of the system (adding more water makes it less viscous). Glass made from freezing foods will be described before discussing how to make them from removing water.
Frozen Glasses

Starting at the solubility limit of sucrose in water at 20 oC is 2 parts sucrose and 1 part water, and once a clear solution forms, cooling begins from (T-1 on the graph) and continues to T2, after which ice will begin to form. During food freezing, water transitions from liquid to ice via nucleation and crystal growth. Water is removed from the unfrozen phase as it forms ice (T-1 to T-2 on the graph), and the unfrozen phase, in effect, becomes more concentrated with solutes.
As the temperature continues to lower (following the phase boundary to T-3), further crystallization proceeds and more ice forms, continually establishing new equilibriums between the concentration of solute in the unfrozen phase and the freezing point depression. Cooling and ice formation continue until the eutectic point (T-3) and the solute sucrose should crystallize. However, crystallization is impeded as the sugar concentration increases drastically, coinciding with an enormous increase in the viscosity of the unfrozen phase (T4 and beyond) when the transition from T3-T4. Beyond the eutectic point, freeze concentration continues until the glass transition is reached, causing a further drastic reduction in molecular mobility. In this case, if more plasticizing molecule (e.g., water) is added, the glass transition decreases to colder temperatures. With current home freezers, frozen glasses foods are impossible at those temperatures. Dipping Dots were the only frozen glassy foods on the market, and they required their distribution chain due to the low glass transition temperatures (~-30oC). If the temperature is not maintained below the glass transition, it becomes soft and sticky; in the case of dipping dots, the perfect sphere clumps together. This state is often termed rubbery as it begins to soften and molecular mobility returns.
Although we have focused on glasses produced with small solutes, glass is also formed with polymers, typically called vitrification. Polymers, such as starch or gelatine, undergo vitrification (glass transition), after which they are completely immobile, producing a very brittle final product (Think dried spaghetti noodles). Near the glass transition is the rubbery transition, which still has a high viscosity; however, the product becomes malleable. Increasing the water content in these products at a constant temperature will significantly reduce the viscosity. Hence it is said that water acts as a plasticizer for polymer glasses. From a food perspective, generally, they contain several components which may pass through the glass transition, such as starches and sugars, in the same material allowing the food to attain the physical properties of a glass much more readily.
Dried or Boiled Glasses

Baking complex food systems obtain stability and structure by removing sufficient water to reach the glass transition of the polymer or solute. Crackers, cookies, milk powders and hard candies all obtain their structure by creating an edible glass. The simplest system is hard candies. Typically, a sucrose solution containing small amounts is boiled to remove water. The sugar solution continually concentrates during boiling until approximately 82% of the final weight, sufficient to produce a sucrose glass when cooled to room temperature. The result is the same as the unfrozen phase glasses discussed above.
Hard candies seem as crystalline solids but lack periodicity and present no crystal lattices. One major problem hard candies encounter is that sugar attracts water to absorb since it is very hygroscopic. Upon absorbing atmospheric water, the concentration of sucrose decreases, and eventually, a state transition occurs from the glassy state to the rubbery state, which causes the product to become sticky. Plasticization into the rubbery is why candies stick together when placed into a dish. Crackers and milk powders are also glasses; however, in this case, they rely on creating a glass due to the presence of polymers, including proteins and long-chain carbohydrates, such as starches. Water is removed from these foods through baking or spray drying, and a glass of the macromolecule is produced through vitrification. Vitrification is why crackers are brittle. Again if water absorbs, it plasticizes the glass, becoming soft and malleable, making them far less desirable and more susceptible to microbial growth. When milk powders or instant coffee powders absorb water, they also become soft, sticky loose viscosity allowing the porous structure to collapse, making them much harder to re-solubilize.


